3-庚基硫砜 | 65016-61-7
中文名称
3-庚基硫砜
中文别名
3-庚基噻吩;3-正庚基噻吩
英文名称
3-heptylthiophene
英文别名
——
CAS
65016-61-7
化学式
C11H18S
mdl
MFCD00130131
分子量
182.33
InChiKey
IUUMHORDQCAXQU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-29.15°C (estimate)
-
沸点:140 °C / 33mmHg
-
密度:0.916
-
LogP:5.544 (est)
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:12
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.636
-
拓扑面积:28.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
储存条件:存放在密封容器内,并放置在阴凉、干燥处。存储地点须远离氧化剂和火源,应置于不易燃区域。
SDS
| Name: | 3-Heptylthiophene 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 65016-61-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 65016-61-7 | 3-Heptylthiophene | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Will burn if involved in a fire. Flammable liquid and vapor.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Remove all sources of ignition.
Use a spark-proof tool.
Section 7 - HANDLING and STORAGE
Handling:
Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Do not breathe dust, vapor, mist, or gas.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 65016-61-7: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear colorless to light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 106-107 deg C @ 3 m
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9160g/cm3
Molecular Formula: C11H18S
Molecular Weight: 182.33
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide, hydrogen sulfide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 65016-61-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Heptylthiophene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 65016-61-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 65016-61-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 65016-61-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:Dithiophene based X-shaped bolaamphiphiles: liquid crystals with single wall honeycombs and geometric frustration摘要:合成了一系列基于5,5′-二苯基-2,2′-二硫代苯的X形聚合物,这些聚合物具有两个长侧链和端部甘油基团,并对这些化合物形成的液晶相进行了偏光显微镜、差示扫描量热法(DSC)和X射线衍射(XRD)研究。这些化合物形成了方形(p4mm和p4gm)和六角形(p6mm)柱状液晶相。在这些中间相中,分子组织成多边形蜂窝结构,π共轭核心形成墙壁,通过甘油单元之间的氢键网络在边缘连接,侧链则填充其中。通过延长这些链,观察到一系列具有“单墙”结构的多边形蜂窝相,形状从三角形到方形、五边形再到六角形。大多数三角形蜂窝似乎是缺陷结构,可以视为带有方向随机的菱形柱体的三角形柱体的混合体。这个不规则的三角形蜂窝到方形蜂窝的转变是通过一个无序的各向同性相进行的。向该各向同性相中添加水会产生一个真正的三角形蜂窝液晶相。用小的甲基取代其中一个长侧链会导致蜂窝形成双墙而不是单墙。紫外线研究表明,芳香核心在蜂窝墙中的堆叠现象,这对这些材料在自组装有机电子材料阵列中的潜在应用具有重要意义。DOI:10.1039/c2sm26575c
-
作为产物:描述:3-(trimethylsilyl)-4-(heptyn-1'-yl)thiophene 在 palladium on activated charcoal 3,4-二溴噻吩 、 四(三苯基膦)钯 、 氢气 、 三氯化硼 、 sodium carbonate 作用下, 以 正己烷 、 三乙胺 为溶剂, 反应 36.0h, 生成 3-庚基硫砜参考文献:名称:3,4-双(三甲基甲硅烷基)噻吩的合成应用:不对称的3,4-二取代噻吩和3,4-二氢噻吩(,)。摘要:3,4-双(三甲基甲硅烷基)噻吩(1a)通过三种途径合成:(a)1,3-偶极环加成;(b)3,4-二溴噻吩的修饰;(c)分子间噻唑-炔Diels-Alder反应。3,4-双(三甲基甲硅烷基)噻吩(1a)可利用其逐步的区域特异性单-ipso取代,然后进行钯催化的交叉偶联反应,来构建不对称的3,4-二取代的噻吩。以这种方式,制备了噻吩15、16、17a-j,19a,b,20、22a-c,23a,b,24a-d,25a-c和27a-j。噻吩-3,4-二基二聚体28和噻吩-3,4-二基四聚体29也是通过钯催化的有机硼氧烷自偶联反应实现的。苯乙烯基噻吩31,通过经由环硼氧烷26c将C-Si键转化为C-Sn键而形成的化合物进行羰基化偶联和锂化,然后用亲电试剂淬灭,从而也提供不对称的3,4-二取代的噻吩33和36a-c。此外,3,4-双(三甲基甲硅烷基)噻吩(1a)可以用作生成高应变环状枯烯3,4-diDOI:10.1021/jo962191n
文献信息
-
Hypervalent iodine(III): selective and efficient single-electron-transfer (SET) oxidizing agent作者:Toshifumi Dohi、Motoki Ito、Nobutaka Yamaoka、Koji Morimoto、Hiromichi Fujioka、Yasuyuki KitaDOI:10.1016/j.tet.2009.10.040日期:2009.12ethers, affording the corresponding aromatic cation radicals. Since then, hypervalent iodine(III) has been utilized as a selective and efficient SET oxidizing agent that enables a variety of direct C–H functionalizations of aromatic rings in electron-rich arenes under mild conditions. We have now extended the original method to work in a series of heteroaromatic compounds such as thiophenes, pyrroles,
-
Cobalt-Catalyzed Reductive Alkylation of Heteroaryl Bromides: One-Pot Access to Alkylthiophenes, -furans, -selenophenes, and -pyrroles作者:Deng-Jhou Cai、Po-Han Lin、Ching-Yuan LiuDOI:10.1002/ejoc.201500784日期:2015.8A practical and convenient Co-catalyzed alkylation method for the facile introduction of various alkyl chains into organic electronically significant heteroaryl compounds, including thiophenes, furans, selenophenes, and pyrroles, is reported. Under well-optimized reaction conditions, a wide range of alkylated heteroaryl compounds have beeen efficiently prepared in moderate to good isolated yields.
-
Continuous Flow Acylation of (Hetero)aryllithiums with Polyfunctional <i>N</i> , <i>N</i> ‐Dimethylamides and Tetramethylurea in Toluene作者:Dimitrije Djukanovic、Benjamin Heinz、Francesca Mandrelli、Serena Mostarda、Paolo Filipponi、Benjamin Martin、Paul KnochelDOI:10.1002/chem.202102805日期:2021.10.7The continuous flow reaction of various aryl or heteroaryl bromides in toluene in the presence of THF (1.0 equiv) with sec-BuLi (1.1 equiv) provided at 25 °C within 40 sec the corresponding aryllithiums which were acylated with various functionalized N,N-dimethylamides including easily enolizable amides at −20 °C within 27 sec, producing highly functionalized ketones in 48–90 % yield (36 examples)
-
Transition between triangular and square tiling patterns in liquid-crystalline honeycombs formed by tetrathiophene-based bolaamphiphiles作者:Xiaohong Cheng、Hongfei Gao、Xiaoping Tan、Xueyan Yang、Marko Prehm、Helgard Ebert、Carsten TschierskeDOI:10.1039/c3sc50664a日期:——self-assemble into a series of liquid-crystalline honeycombs, formed by the π-conjugated rods which enclose polygonal prismatic cells filled by the lateral chains. With increasing chain length a discontinuous transition from triangular to square honeycombs takes place. At this transition a periodic honeycomb composed of a mixture of square and triangular cells in a ratio 1 : 2 was formed at low temperature, whereas
-
The synthesis of head-to-tail (H–T) dimers of 3-substituted thiophenes by the hypervalent iodine(iii)-induced oxidative biaryl coupling reaction作者:Toshifumi Dohi、Koji Morimoto、Yorito Kiyono、Akinobu Maruyama、Hirofumi Tohma、Yasuyuki KitaDOI:10.1039/b503058g日期:——The head-to-tail (H-T) dimers could be obtained selectively by the oxidative coupling reaction of 3-substituted thiophenes using a combination of hypervalent iodine(III) reagents and trimethylsilyl trifluoromethanesulfonate.
表征谱图
-
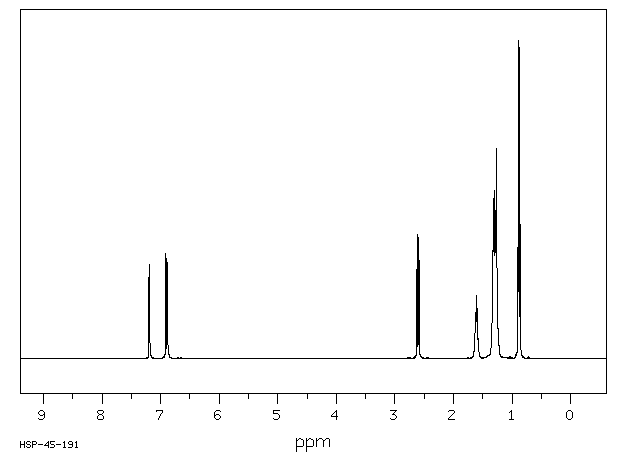
氢谱1HNMR
-
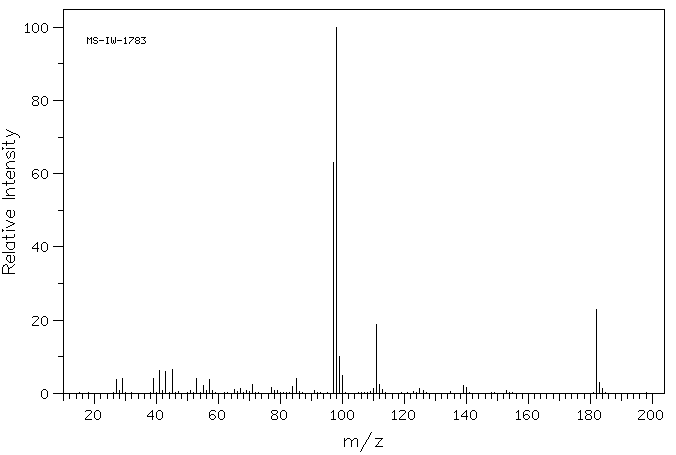
质谱MS
-
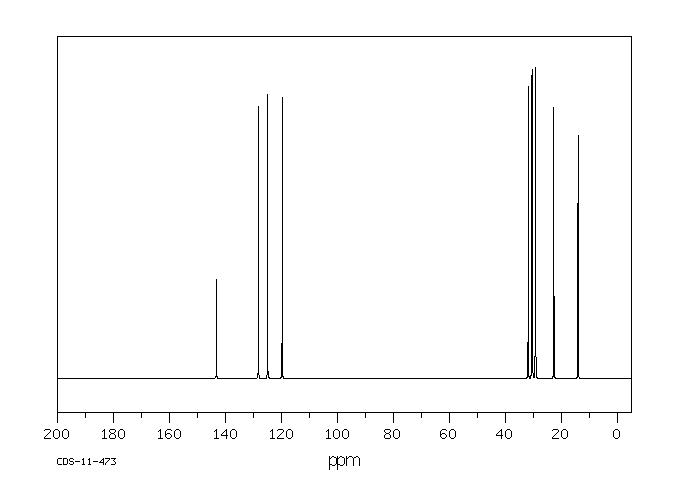
碳谱13CNMR
-
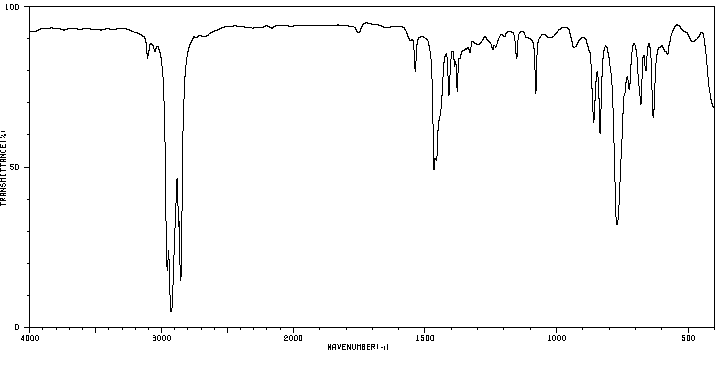
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
香薷二醇
顺式-1-(2-呋喃基)-1-戊烯
顺-1,2-二氰基-1,2-双(2,4,5-三甲基-3-噻吩基)乙烯
顺-1,2-(2-噻嗯基)二乙烯
雷尼替丁-N,S-二氧化物
雷尼替丁-N-氧化物
钴(II)双[(2-吡啶基甲基)(叔丁基二甲基甲硅烷基)酰胺]
西拉诺德
螺[环氧乙烷-2,3'-吡咯并[1,2-a]吡嗪]
萘并[2,1,8-def]喹啉
苯硫基溴化镁
苯甲酸,2-[[[7-[[(3.β.)-3-羟基-28-羰基羽扇-20(29)-烯-28-基]amino]庚基]氨基]羰基]
苍术素
羟胺,O-[4-(2-呋喃基)丁基]-
缩水甘油糠醚
紫苏烯
糠醛肟
糠醛氰醇的1-乙氧基乙基醚
糠醇-d2
糠醇
糠基硫醇-d2
糠基硫醇
糠基甲基硫醚
糠基氯
糠基氨基甲酸异丙酯
糠基丙基醚
糠基丙基二硫醚
糠基3-巯基-2-甲基丙酸酯
糠基-异戊基醚
糠基-异丁基醚
糠基 2-甲基-3-呋喃基二硫醚
磷杂茂
碘化N,N,N-三甲基丁烷-1-铵
硫酸异丙基糠酯
硫代磷酸O-糠基O-甲基S-(2-丙炔基)酯
硫代磷酸O-乙基O-糠基S-(2-丙炔基)酯
硫代甲酸S-糠酯
硫代噻吩甲酰基三氟丙酮
硫代乙酸糠酯
硫代丙酸糠酯
硒吩-3-羧酸酰肼
硅烷,三(1-甲基乙基)[(3-甲基-2-呋喃基)氧代]-
硅烷,[2-(3-呋喃基)乙烯基]三甲基-,(E)-
硅烷,(1,1-二甲基乙基)(2-呋喃基甲氧基)二甲基-
砷杂苯
甲酸糠酯
甲氧亚胺基呋喃乙酸铵盐
甲基糠基醚
甲基糠基二硫
甲基呋喃-2-基甲基氨基甲酸酯