4-羟基-2-甲基吲哚 | 35320-67-3
中文名称
4-羟基-2-甲基吲哚
中文别名
——
英文名称
4-hydroxy-2-methyl-1H-indole
英文别名
4-hydroxy-2-methylindole;2-methyl-1H-indol-4-ol;2-methyl-4-hydroxyindole
CAS
35320-67-3
化学式
C9H9NO
mdl
MFCD00152133
分子量
147.177
InChiKey
XBVSGEGNQZAQPM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:122-124°C
-
沸点:339.2±22.0 °C(Predicted)
-
密度:1.262±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
稳定性/保质期:
常规情况下不会分解,也没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.111
-
拓扑面积:36
-
氢给体数:2
-
氢受体数:1
安全信息
-
危险品标志:Xn,Xi,T
-
WGK Germany:3
-
RTECS号:KS0250000
-
海关编码:2933990090
-
安全说明:S24/25,S26,S36,S36/37
-
危险类别码:R20/21/22,R36/37/38
-
包装等级:III
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封保存于阴凉干燥处
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
4-Hydroxy-2-methylindole
Product Name:
Synonyms: 2-Methyl-1H-indol-4-ol
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
4-Hydroxy-2-methylindole
Ingredient name:
CAS number: 35320-67-3
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H9NO
Molecular weight: 147.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
4-Hydroxy-2-methylindole
Product Name:
Synonyms: 2-Methyl-1H-indol-4-ol
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
4-Hydroxy-2-methylindole
Ingredient name:
CAS number: 35320-67-3
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H9NO
Molecular weight: 147.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-methyl-4-(oxiran-2-ylmethoxy)-1H-indole 62119-47-5 C12H13NO2 203.241 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— [(2-methyl-1H-indol-4-yl)oxy]acetic acid 359586-05-3 C11H11NO3 205.213 —— methyl [(2-methyl-1H-indol-4-yl)oxy]acetate 359586-04-2 C12H13NO3 219.24 —— (2S)-2-methylindol-4-yl glycidyl ether 256373-18-9 C12H13NO2 203.241 —— ethyl (2-methyl-1H-indol-4-yloxy)acetate 1000606-03-0 C13H15NO3 233.267 —— allyl [(2-methyl-1H-indol-4-yl)oxy]acetate 359586-06-4 C14H15NO3 245.278 —— 2-methyl-1H-indol-4-yloxyphenylmethyl acetic acid benzyl ester 502434-65-3 C18H17NO3 295.338 —— 2-methyl-1H-indol-4-yl trifluoromethanesulfonate 359585-96-9 C10H8F3NO3S 279.24 —— 3-methyl-2-(2-methyl-1H-indol-4-yloxy)-butyric acid ethyl ester 936617-26-4 C16H21NO3 275.348 —— tert-butyl 3-methyl-2-[(2-methyl-1H-indol-4-yl)oxy]butanoate 936567-89-4 C18H25NO3 303.401
反应信息
-
作为反应物:描述:4-羟基-2-甲基吲哚 在 氨 、 potassium carbonate 作用下, 以 二氯甲烷 、 丙酮 为溶剂, 反应 14.17h, 生成 tert-butyl ({3-[amino(oxo)acetyl]-2-methyl-1H-indol-4-yl}oxy)acetate参考文献:名称:The First Potent Inhibitor of Mammalian Group X Secreted Phospholipase A2: Elucidation of Sites for Enhanced Binding摘要:Using the X-ray structure of human group X secreted phospholipase A(2) (hGX), we carried out structure-based design of indole-based inhibitors and prepared the compounds using a new synthetic route. The most potent compound inhibited hGX and the mouse orthologue with an IC50 of 75 nM. This compound is the most potent hGX inhibitor reported to date and was also found to inhibit a subset of the other mouse and human sPLA(2)s.DOI:10.1021/jm060136t
-
作为产物:描述:7-[3-(苄基氨基)丙基]-3,7-二氢-1,3-二甲基-1H-嘌呤-2,6-二酮 、 2-methyl-4-(oxiran-2-ylmethoxy)-1H-indole 以obtained from 5.9 g of 2-methyl-4-hydroxy indole的产率得到4-羟基-2-甲基吲哚参考文献:名称:Basic substituted xanthine derivatives摘要:有准备好的化合物对应于一般式T-Alk-NH-CH.sub.2-CH(OH)-CH.sub.2-O-Ar I,在其中T代表茶碱基-(7)或可可碱基-(1)基团,Alk是具有2到5个碳原子的线性或支链烷基,也可以被一个羟基取代,Ar代表一个芳香单环或紧缩的双环、碳环或杂环基团,可选择地被一个或多个具有1到6个碳原子的烷基、具有2到6个碳原子的烯基、具有2到6个碳原子的炔基、羟基、具有1到6个碳原子的酰氧基、具有1到6个碳原子的烷氧基、具有2到6个碳原子的烯氧基、苯基基团、卤原子、氨基、具有2到6个碳原子的酰基、氨基甲酰基、脲基、具有1到6个碳原子的酰氨基基团、具有3到8个碳原子的环烷基或一个或多个具有4到8个碳原子的环烯基基团取代,杂环基团由5到6个成员的单独环组成,并含有1到4个杂原子,以及它们的药学上可接受的盐。这些化合物具有β-肾上腺素受体拮抗活性。公开号:US04144340A1
文献信息
-
[EN] PYRIMIDINE INDOLE DERIVATIVES FOR TREATING CANCER<br/>[FR] DÉRIVÉS DE PYRIMIDINE ET D'INDOLE POUR LE TRAITEMENT DU CANCER申请人:ASTRAZENECA AB公开号:WO2010073034A1公开(公告)日:2010-07-01There is provided pyrimidinyl indole compounds of Formula (I), or pharmaceutically acceptable salts thereof, processes for their preparation, pharmaceutical compositions containing them and their use in therapy, particularly for treating cancer.
-
NOVEL 6-5 BICYCIC HETEROCYCLIC DERIVATIVE AND MEDICAL USE THEREOF申请人:Sanwa Kagaku Kenkyusho Co., Ltd公开号:EP2036887A1公开(公告)日:2009-03-18An object of the present invention is to provide a medicament as a thyroid hormone receptor ligand which is sufficient in drug efficacy and safety, and has the excellent action as a drug. The present invention provides a compound represented by the following general formula (I) or a pharmaceutically acceptable salt thereof: [wherein [Chemical Formula 2] is a single bond or a double bond; A is -CH2- or -CO-; X, Y, and Z are each independently a nitrogen atom or a carbon atom; R1 is a hydrogen atom or an aralkyl group; R2 is an alkyl group or an aralkyl group, etc.; R3 is a hydrogen atom or an alkyl group, etc.; R4 is a hydrogen atom or an alkyl group; R5 is a hydrogen atom, an alkyl group or a halo lower alkyl group, etc.; R6 is a hydrogen atom or an alkyl group; R7 is a hydrogen atom, etc.; R8 is a hydrogen atom, or an alkyl group, etc.; and E is -NHCO-G-COR12, etc. (wherein G is a single bond or an alkylene group, and R12 is a hydroxy group or an alkoxy group)].
-
[EN] MONO AND BICYCLIC RING BORONIC ACID, ESTER AND SALT COMPOUNDS AS INHIBITORS OF P97 COMPLEX<br/>[FR] COMPOSÉS D'ESTER, DE SEL ET D'ACIDE BORONIQUE À NOYAU MONOCYCLIQUE ET BICYCLIQUE EN TANT QU'INHIBITEURS DE COMPLEXE P97申请人:CLEAVE BIOSCIENCES INC公开号:WO2016200840A1公开(公告)日:2016-12-15Monocyclic or bicyclic ring boronic acid or ester compounds having an arylalkyi amine substituent at the 4 position and a substituted 5:6 bicyclic group at the 2 position of the mono or bicyclic ring boronic acid or ester as well as optional aliphatic, functional and/or aromatic components substituted at other positions of the mono or bicyclic boronic acid or ester compounds are disclosed. These compounds are inhibitors of the AAA proteasome complex containing p97 and are effective medicinal agents for treatment of diseases associated with the Valosin containing protein and p97 bioactivity such as cancer. Formula (I).
-
[EN] SUBSTITUTED TRICYCLIC AMIDES, ANALOGUES THEREOF, AND METHODS USING SAME<br/>[FR] AMIDES TRICYCLIQUES SUBSTITUÉS, ANALOGUES DE CEUX-CI ET PROCÉDÉS LES METTANT EN OEUVRE申请人:ARBUTUS BIOPHARMA CORP公开号:WO2021229302A1公开(公告)日:2021-11-18The present disclosure includes substituted tricyclic amides, or analogues thereof of formula (I) (I), wherein X, Y, ring A, R1, R5, R6 and R7 are as defined herein, and compositions comprising compounds of formula (I) that can be used to treat or prevent hepatitis B virus (HBV) and/or hepatitis D virus (HDV) infections in a patient.
-
Indole derivatives for the treatment of depression and anxiety申请人:——公开号:US20040006229A1公开(公告)日:2004-01-08The present invention provides compounds of formula (I): which are useful for treating depression, anxiety, and alleviating the symptoms caused by withdrawal or partial withdrawal from the use of tobacco or of nicotine. 1本发明提供了以下化合物的公式(I):这些化合物对治疗抑郁症、焦虑症,并缓解因戒烟或部分戒烟引起的症状非常有用。
表征谱图
-
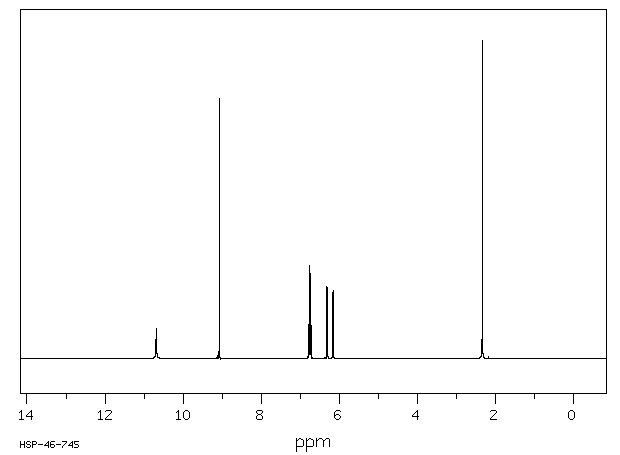
氢谱1HNMR
-
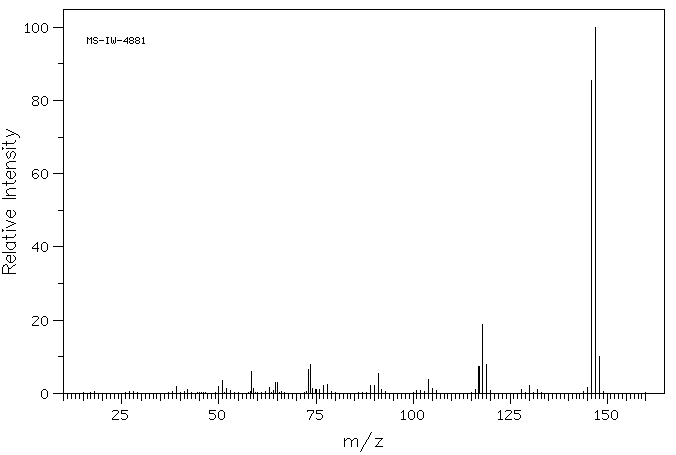
质谱MS
-
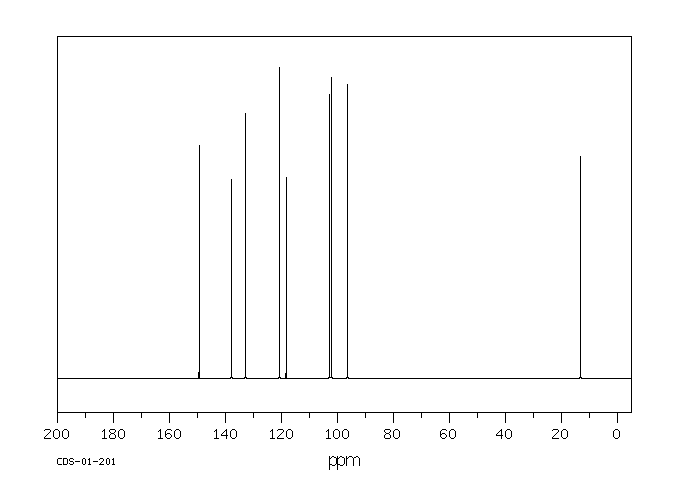
碳谱13CNMR
-
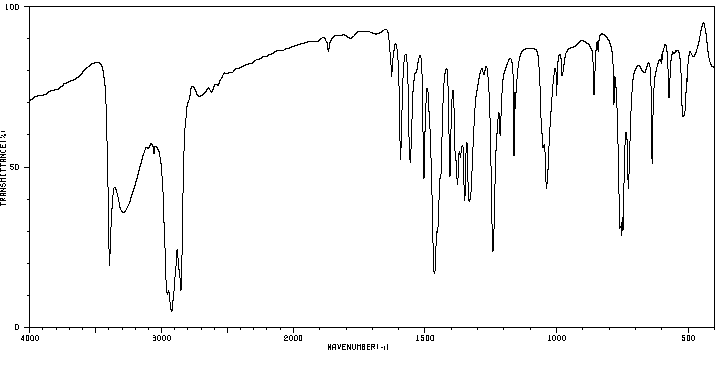
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3