1,1,2-三氯丙烷 | 598-77-6
中文名称
1,1,2-三氯丙烷
中文别名
——
英文名称
1,1,2-trichloropropane
英文别名
112TCP;1,1,2-trichloro-propane;1,1,2-Trichlor-propan;α-Chlorpropylidenchlorid;1,1,3-Trichlor-propan;1,1,2-Trichlorpropan
CAS
598-77-6
化学式
C3H5Cl3
mdl
MFCD00018864
分子量
147.432
InChiKey
GRSQYISVQKPZCW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-36.84°C (estimate)
-
沸点:133 °C
-
密度:1.35
-
溶解度:0.01 M
-
蒸汽压力:3.10 mmHg
-
保留指数:827;827
-
稳定性/保质期:
性质与稳定性:在常温常压下,不会发生分解反应。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险类别码:R20/21/22,R36/37/38
-
储存条件:贮存: 将密器密封后,储存在密封的主要容器中,并放置在阴凉、干燥的地方。
SDS
Section I.Chemical Product and Company Identification
Chemical Name 1,1,2-Trichloropropane
Portland OR
Synonym Propane, 1,1,2-trichloro- (CA INDEX NAME);
2-Chloropropylidene Chloride
Chemical Formula C3H5Cl3
CAS Number 598-77-6
Section II. Composition and Information on Ingredients
Toxicology Data
Chemical Name CAS Number Percent (%) TLV/PEL
Min. 96.0 (GC) Not available. Rat LD50 (oral) 1230 mg/kg
1,1,2-Trichloropropane 598-77-6
Rabbit LD50 (dermal) 14100 uL/kg
Section III. Hazards Identification
Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Acute Health Effects
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability Combustible.
Flammable Limits Not available.
Flash Points Not available.
Combustion Products These products are toxic carbon oxides (CO, CO2), halogenated compounds.
WARNING: Highly toxic HCl gas is produced during combustion.
Fire Hazards Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
1,1,2-Trichloropropane
Section VI. Accidental Release Measures
Spill Cleanup Combustible material. Harmful material. Irritating material.
Keep away from heat. Mechanical exhaust required. Stop leak if without risk. Finish cleaning the spill by rinsing any
Instructions
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
COMBUSTIBLE. HARMFUL. IRRITANT. Keep away from heat. Mechanical exhaust required. Avoid excessive heat and
Handling and Storage
light. Do not breathe gas/fumes/ vapor/spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their respective
Engineering Controls
threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
Personal Protection
specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Liquid. (Clear, colorless.) Solubility
Physical state @ 20°C Not available.
1.35 (water=1)
Specific Gravity
Molecular Weight 147.43 Partition Coefficient
Not available.
Boiling Point 133°C (271.4°F) Vapor Pressure Not available.
Not available. Not available.
Melting Point Vapor Density
Refractive Index 1.4670 - 1.4700 Volatility Not available.
Not available. Not available.
Critical Temperature Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
This material is stable if stored under proper conditions. (See Section VII for instructions)
Stability
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
TZ8925000
RTECS Number
Eye Contact. Ingestion. Inhalation.
Routes of Exposure
Rat LD50 (oral) 1230 mg/kg
Toxicity Data
Rabbit LD50 (dermal) 14100 uL/kg
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Acute Toxic Effects
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye
is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Continued on Next Page
1,1,2-Trichloropropane
Section XIII. Disposal Considerations
Waste Disposal Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list.
(EPA)
WHMIS Classification CLASS B-3: Combustible liquid with a flash point between 37.8°C (100°F) and 93.3°C (200°F).
On NDSL
(Canada)
EINECS Number (EEC) 209-951-8
EEC Risk Statements R20/21/22- Harmful by inhalation, in contact with skin and if swallowed.
R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2-二氯丙烷 1,2-Dichloropropane 78-87-5 C3H6Cl2 112.987 1,1-二氯丙烷 1,1-dichloropropane 78-99-9 C3H6Cl2 112.987 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 四氯丙烷 1,1,2,3-tetrachloropropane 18495-30-2 C3H4Cl4 181.877 五氯丙烷 1,1,2,2,3-pentachloropropane 16714-68-4 C3H3Cl5 216.322 —— 1,1,1,2,2-pentachloropropane 64240-29-5 C3H3Cl5 216.322 1,2,3-三氯丙烷 1,2,3-trichloropropane 96-18-4 C3H5Cl3 147.432 六氯丙烷 1,1,2,2,3,3-Hexachlorpropan 15600-01-8 C3H2Cl6 250.767
反应信息
-
作为反应物:描述:参考文献:名称:McBee et al., Journal of the American Chemical Society, 1947, vol. 69, p. 946摘要:DOI:
-
作为产物:描述:参考文献:名称:[EN] PROCESS FOR THE PRODUCTION OF CHLORINATED ALKANES
[FR] PROCÉDÉ DE PRODUCTION D'ALCANES CHLORÉS摘要:提供了生产氯代烷的工艺。现有工艺包括使用催化剂体系催化含有一种或多种烷烃和/或烯烃的进料流的氯化反应,所述催化剂体系包括一种或多种无机碘盐和/或低于常规水平的元素碘和至少一种路易斯酸。该工艺在非水介质中进行,因此,一种或多种无机碘盐可以全部或部分回收和/或重复使用。公开号:WO2013082410A1 -
作为试剂:描述:4-bromo-3,6-difluoro-2-nitroaniline 在 1,1,2-三氯丙烷 、 palladium on activated charcoal 、 氢气 作用下, 以 甲醇 、 水 为溶剂, 25.0 ℃ 、206.85 kPa 条件下, 以93.4%的产率得到3,6-difluorobenzene-1,2-diamine hydrochloride参考文献:名称:[EN] NOVEL COMPOUNDS
[FR] NOUVEAUX COMPOSÉS摘要:本发明提供了式(I)的化合物及其药学上可接受的盐,N-氧化物,溶剂合物和前药。式(I)中,R2,R3,R4,X1,X2,X3和X4如规范中所定义,制备它们的过程,含有它们的制药组合物及其在治疗中的应用,特别是用于治疗与KCNK13活性相关的疾病。公开号:WO2022167819A1
文献信息
-
Compounds And Compositions for the Treatment of Ocular Disorders申请人:Graybug Vision, Inc.公开号:US20170080092A1公开(公告)日:2017-03-23The disclosure describes prodrugs and derivatives of prostaglandins, carbonic anhydrase inhibitors, kinase inhibitors, beta-adrenergic receptor antagonists and other drugs, as well as controlled delivery formulations containing such prodrugs and derivatives, for the treatment of ocular disorders.
-
[EN] PROCESS FOR THE PRODUCTION OF CHLORINATED PROPENES<br/>[FR] PROCÉDÉ POUR LA PRODUCTION DE PROPÈNES CHLORÉS申请人:DOW GLOBAL TECHNOLOGIES LLC公开号:WO2012166394A1公开(公告)日:2012-12-06Processes for the production of chlorinated propenes are provided. The present processes make use of 1,2-dichloropropane, a by-product in the production of chlorohydrin, as a low cost starting material, alone or in combination with 1,2,3-trichloropropane. The present processes can also generate anhydrous HCl as a byproduct that can be removed from the process and used as a feedstock for other processes, providing further time and cost savings. Finally, the processes are advantageously conducted in the liquid phase, thereby presenting additional savings as compared to conventional, gas phase processes.
-
HETEROCYCLIC COMPOUNDS AND THEIR USE AS GLYCOGEN SYNTHASE KINASE-3 INHIBITORS申请人:Turner Sean Colm公开号:US20120172376A1公开(公告)日:2012-07-05The present invention relates to novel heterocyclic compounds of formula I wherein the variables are as defined in the claims or the description, which are useful for inhibiting glycogen synthase kinase 3 (GSK-3), compositions containing the compounds, their use for preparing a medicament for the treatment of a medical disorder susceptible to the treatment with a compound that modulates, preferably inhibits, the activity of glycogen synthase kinase 3β, and methods of treatment of medical disorders susceptible to treatment with a compound that modulates glycogen synthase kinase 3β activity using the compounds.
-
[EN] SULFURYL CHLORIDE AS CHLORINATING AGENT<br/>[FR] CHLORURE DE SULFURYLE COMME AGENT CHLORANT
-
[EN] PROCESS FOR THE PRODUCTION OF CHLORINATED AND/OR BROMINATED PROPANES AND/OR PROPENES<br/>[FR] PROCÉDÉ DE PRODUCTION DE PROPANES ET/OU PROPÈNES CHLORÉS ET/OU BROMÉS申请人:DOW GLOBAL TECHNOLOGIES LLC公开号:WO2013090354A1公开(公告)日:2013-06-20Processes for the production of chlorinated and/or brominated propanes and/or propenes are provided. The present processes make use of a feedstream comprising 1,2-dichloropropane and at least one bromination step. Regioselectivities of at least 90% to desired dichlorobromopropanes are provided. The present processes may also include one or more halogen exchange and/or dehydrohalogenation steps that leverage the regioselectivities provided by the initial bromination step.
表征谱图
-
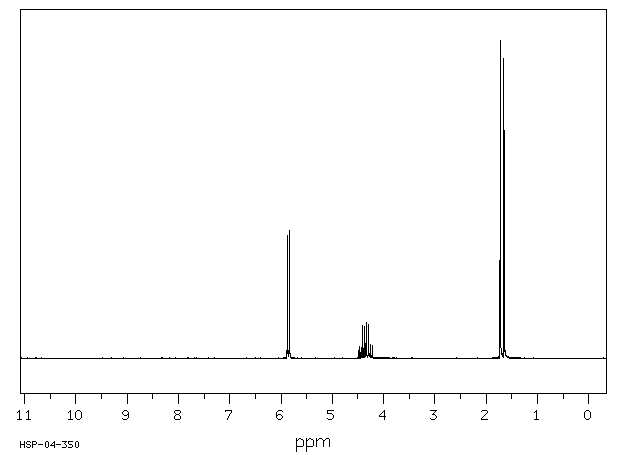
氢谱1HNMR
-
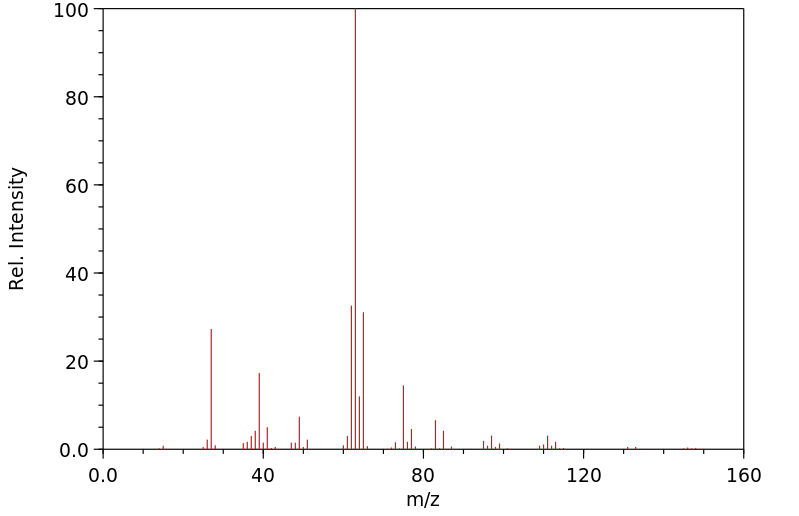
质谱MS
-
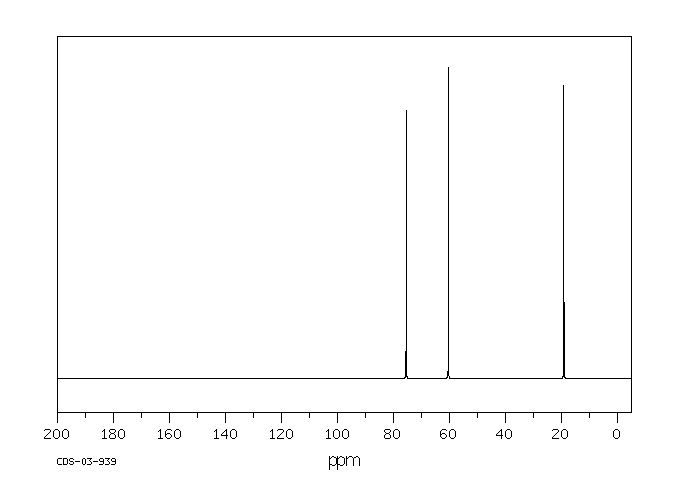
碳谱13CNMR
-
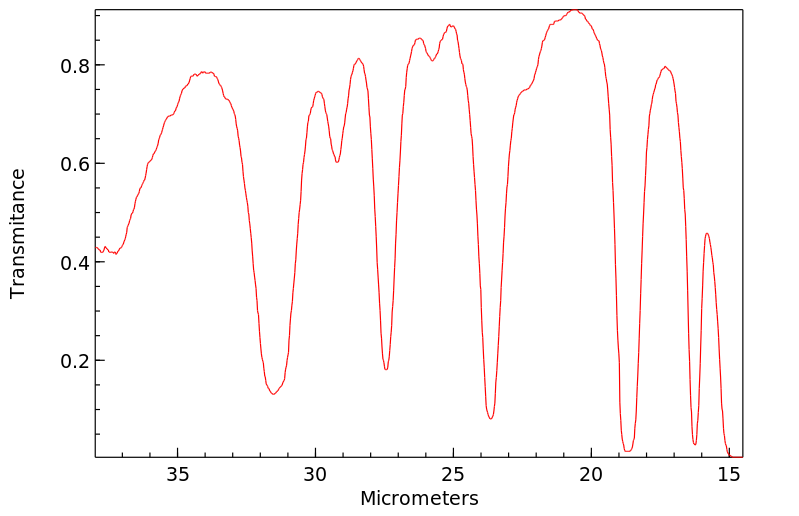
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷