2,2,2-三氟-2',4',6'-三甲基苯乙酮 | 313-56-4
中文名称
2,2,2-三氟-2',4',6'-三甲基苯乙酮
中文别名
2,2,2-三氟-2,4,6-三甲基苯乙酮
英文名称
α,α,α-trifluoro-2,4,6-trimethylacetophenone
英文别名
2,4,6-trimethyl-α,α,α-trifluoroacetophenone;2',4',6'-trimethyl-2,2,2-trifluoroacetophenone;2',4',6'-trimethyltrifluoroacetophenone;trifluoroacetylmesitylene;2,2,2-trifluoro-1-(2,4,6-trimethylphenyl)-ethanone;2,2,2-Trifluoro-2',4',6'-trimethylacetophenone;2,2,2-trifluoro-1-(2,4,6-trimethylphenyl)ethanone
CAS
313-56-4
化学式
C11H11F3O
mdl
——
分子量
216.203
InChiKey
VINRTVDNUHIWCB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:70-80 °C20 mm Hg(lit.)
-
密度:1.136 g/mL at 25 °C(lit.)
-
闪点:170 °F
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,也不存在已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:15
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:F,Xi
-
危险类别码:R36/37/38
-
海关编码:2914700090
-
安全说明:S26,S36
-
储存条件:请将贮藏器密封并存放在阴凉干燥处,同时确保工作环境有良好的通风或排气设施。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
: 2,2,2-Trifluoro-2′,4′,6′-trimethylacetophenone
Product name
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 313-56-4
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Skin irritation (Category 2), H315
Eye irritation (Category 2), H319
Specific target organ toxicity - single exposure (Category 3), H335
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xi Irritant R36/37/38
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Warning
Hazard statement(s)
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Formula : C11H11F3O
Molecular Weight : 216,20 g/mol
CAS-No. : 313-56-4
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
2,2,2-Trifluoro-2',4',6'-trimethylacetophenone
CAS-No. 313-56-4 Skin Irrit. 2; Eye Irrit. 2; STOT <= 100 %
SE 3; H315, H319, H335
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
2,2,2-Trifluoro-2',4',6'-trimethylacetophenone
CAS-No. 313-56-4 Xi, R36/37/38 <= 100 %
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
For small (incipient) fires, use media such as "alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large quantities (flooding) of water applied as a mist or
spray; solid streams of water may be ineffective. Cool all affected containers with flooding quantities of
water.
Special hazards arising from the substance or mixture
Carbon oxides, Hydrogen fluoride
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
Use water spray to cool unopened containers.
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapours, mist or gas. Ensure adequate ventilation.
Remove all sources of ignition. Evacuate personnel to safe areas. Beware of vapours accumulating to
form explosive concentrations. Vapours can accumulate in low areas.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Contain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and
place in container for disposal according to local regulations (see section 13). Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Keep away from sources of ignition - No smoking.Take measures to prevent the build up of electrostatic
charge.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: liquid
Colour: colourless
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 70 - 80 °C at 27 hPa - lit.
boiling range
g) Flash point 77 °C - closed cup
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,136 g/cm3 at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
Heat, flames and sparks.
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
This combustible material may be burned in a chemical incinerator equipped with an afterburner and
scrubber. Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(2,4,6-三甲基苯基)-2,2,2-三氟乙醇 1-(2,4,6-trimethylphenyl)-2,2,2-trifluoroethanol 102626-53-9 C11H13F3O 218.219 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (R)-2,2,2-trifluoro-1-(2,4,6-trimethylphenyl)ethanol 102626-53-9 C11H13F3O 218.219 —— (S)-2,2,2-trifluoro-1-(2,4,6-trimethylphenyl)ethanol 248245-58-1 C11H13F3O 218.219
反应信息
-
作为反应物:描述:2,2,2-三氟-2',4',6'-三甲基苯乙酮 在 oxazaborolidine 1 (R=n-Bu) 、 儿萘酚硼烷 作用下, 以 甲苯 为溶剂, 反应 24.0h, 以100%的产率得到(R)-2,2,2-trifluoro-1-(2,4,6-trimethylphenyl)ethanol参考文献:名称:从非手性酮中合成对映体纯的仲甲醇非常有效和简单摘要:手性恶唑硼烷(1)催化还原酮的2,6和8提供了简单访问手性仲原醇3,7,和9以非常高的对映体纯度。DOI:10.1016/0040-4039(91)80419-7
-
作为产物:描述:1-(2,4,6-三甲基苯基)-2,2,2-三氟乙醇 在 KetoABNO 、 氧气 、 溶剂黄146 、 sodium nitrite 作用下, 20.0 ℃ 、101.33 kPa 条件下, 反应 18.0h, 以70%的产率得到2,2,2-三氟-2',4',6'-三甲基苯乙酮参考文献:名称:室温下α-氟代烷基醇的有机催化好氧氧化为氟代烷基酮摘要:描述了缺电子的α-氟代烷基醇在室温下的有机催化好氧氧化。所得的氟代烷基酮是多种含氟分子的通用合成中间体。现在,这种困难的转化是通过α-氟代烷基醇与N-氧基自由基的反应完成的,该自由基是由9-氮杂双环[3.3.1]壬基-3-酮N-氧基/氮氧化物(酮基ABNO / NO)催化生成的X)和氧气在乙酸(AcOH)中的反应,以高收率得到相应的氟代烷基酮。该操作简单的反应可以在温和的条件下进行,并且已应用于多种醇(20种实例),因此证明了对官能团的高耐受性。此外,基于此方法的改进的一锅操作规程能够将克转化为醛,将醛转化为三氟甲基酮。DOI:10.1002/adsc.201500131
文献信息
-
Fine tuning of molecular and supramolecular properties of simple trianglimines – the role of the functional group作者:J. Gajewy、J. Szymkowiak、M. KwitDOI:10.1039/c6ra06095a日期:——affected by the nature and chirality of the dopant. The hexaimine macrocycles after reduction of the CN imine bonds formed trianglamines – useful chiral ligands in stereoselective synthesis. The Zn–trianglamine complexes were employed as catalysts for asymmetric hydrosilylation of prochiral ketones, providing products of enantiomeric excess up to 98%. This remains the best result obtained for Zn–diamine容易从对映体纯的反式-1,2-二氨基环己烷和各种芳族二醛中获得的手性,三角形聚氮杂大环(trianglimines),其性质和取代方式不同。在满足所谓的对称规则的熵的热力学控制下,形成具有两个连接于芳基部分的给电子基团的高度对称的大环。相反,仅从2-硝基对苯二甲酸乙醛获得平凡的C 1对称性的动力学产物,而从2-甲氧基对苯二甲酸乙醛则得到C 3-和C 1的混合物。对称的大环形成。在实验/理论方法的基础上确定了影响大环形成机理的因素。大环的非对称结构是由环缩合过程中出现的对称中间体引起的。通过量子化学计算支持的实验性ECD和VCD方法研究了Trianglimines的手性。硝基取代的trianglimine似乎是在稳定的手性有机凝胶的极性介质中形成的一种简单的低分子量超级胶凝剂。凝胶的结构受掺杂剂的性质和手性的影响。C还原后的六亚胺大环N亚胺键形成trianglamines –在立体选择性合成
-
Asymmetric hydrosilylation of ketones catalyzed by complexes formed from trans-diaminocyclohexane-based diamines and diethylzinc作者:Jadwiga Gajewy、Jacek Gawronski、Marcin KwitDOI:10.1007/s00706-012-0754-0日期:2012.7Chiral acyclic and macrocyclic amines derived from trans-1,2-diaminocyclohexane in complexes with diethylzinc efficiently catalyze asymmetric hydrosilylation of aryl-alkyl and aryl-aryl ketones with enantiomeric excess of the product up to 86 %. A trianglamine ligand with a cyclic structure or the presence of an additional coordinating group increases the enantioselectivity of the reaction, in comparison摘要:由反式 1,2-二氨基环己烷衍生的手性无环胺和大环胺与二乙基锌形成络合物,可有效催化芳基烷基酮和芳基芳基酮的不对称氢化硅烷化,产物对映体过量高达 86%。与简单的无环N,N'-二苄基-1,2-二氨基环己烷配体的催化相比,具有环状结构或存在额外配位基团的三胺配体提高了反应的对映选择性。此外,还研究了多种醇和二醇对催化剂不对称活化的影响。图形概要:
-
Cyanide-ion-catalyzed reaction of pentafluorophenyltrimethylsilane with substituted acetophenones作者:O.A. Vyazankina、B.A. Gostevskii、N.S. VyazankinDOI:10.1016/0022-328x(85)87329-0日期:1985.9Treatment of pentafluorophenyltrimethylsilane (I) with enolizable ketones in the presence of a catalytic amount of potassium cyanide-18-crown-6 complex gave the corresponding trimethylsilyl enol ethers. The same dehydrogenative silylation of highly crowded 2,4,6-trimethylacetophenone with silane I or with Me3SiCN was extended to the preparation of 1′-(2,4,6-trimethylstyryl)oxytrimethylsilane (XII)
-
Direct Synthesis of a Trifluoromethyl Copper Reagent from Trifluoromethyl Ketones: Application to Trifluoromethylation作者:Hiroki Serizawa、Kohsuke Aikawa、Koichi MikamiDOI:10.1002/chem.201303828日期:2013.12.23fluorine: The direct synthesis of CuCF3 from a cuprate reagent and trifluoromethyl ketones, as one of the most economical and efficient trifluoromethyl sources, was accomplished. The advantages of this method are all of reagents employed are low‐cost, operation is simple, and the yield of CuCF3 is virtually quantitative (see scheme). Furthermore, three types of trifluoromethylations smoothly proceeded
-
Trifluoromethyl Oxetanes: Synthesis and Evaluation as a <i>tert</i> -Butyl Isostere作者:Paramita Mukherjee、Martin Pettersson、Jason K. Dutra、Longfei Xie、Christopher W. am EndeDOI:10.1002/cmdc.201700333日期:2017.10.9The synthesis of a new trifluoromethyl oxetane was developed using a Corey–Chaykovsky epoxidation/ring‐expansion reaction of trifluoromethyl ketones. The reaction was shown to proceed under mild conditions and displays a broad substrate scope. The trifluoromethyl oxetane was also evaluated as a tert‐butyl isostere in the context of the γ‐secretase modulator (GSM) program. We demonstrate that the trifluoromethyl
表征谱图
-
氢谱1HNMR
-
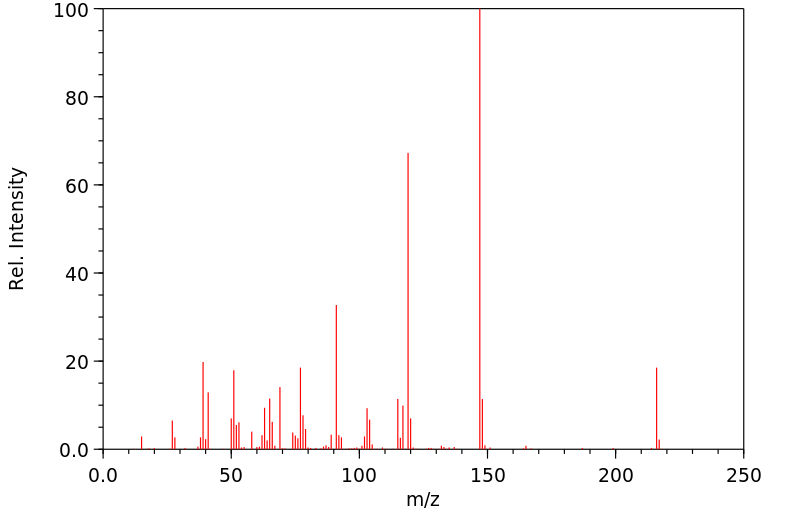
质谱MS
-
碳谱13CNMR
-
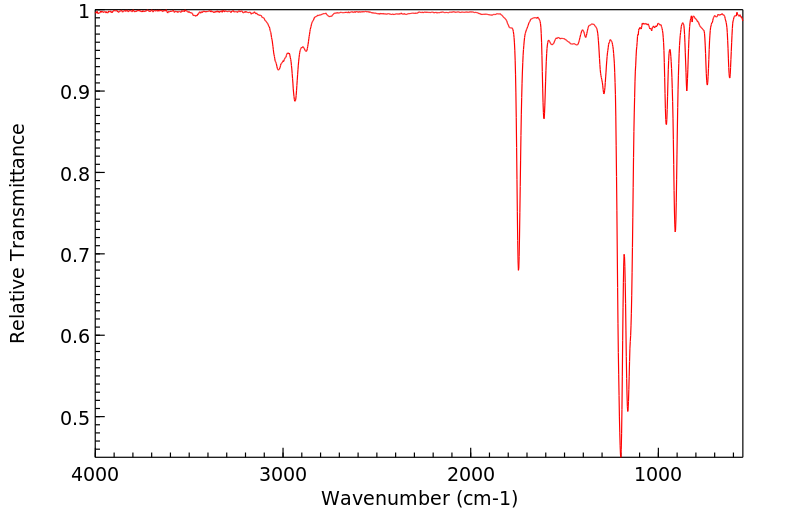
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷