2,2-二甲基-3-庚酮 | 19078-97-8
中文名称
2,2-二甲基-3-庚酮
中文别名
——
英文名称
2,2-dimethyl-3-heptanone
英文别名
2,2-dimethylheptan-3-one
CAS
19078-97-8
化学式
C9H18O
mdl
——
分子量
142.241
InChiKey
ZLMHETMAEHQFHK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:164 °C(Press: 735 Torr)
-
密度:0.823 g/cm3(Temp: 11 °C)
-
保留指数:964.7;965;963;963;965
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.89
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2914190090
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P210,P240,P241,P242,P243,P261,P264,P271,P280,P302+P352,P303+P361+P353,P304+P340,P305+P351+P338,P312,P332+P313,P337+P313,P362,P370+P378,P403+P233,P403+P235,P405,P501
-
危险品运输编号:1224
-
危险性描述:H225,H315,H319,H335
-
储存条件:2-8°C
SDS
上下游信息
反应信息
-
作为反应物:描述:2,2-二甲基-3-庚酮 在 lithium aluminium tetrahydride 作用下, 生成 2,2-二甲基-3-庚醇参考文献:名称:Valproate-Associated Stomatitis摘要:这篇文章的目的是报告一例患有小儿癫痫并服用丙戊酸钠的患者出现严重口腔炎的病例。该病例通过详细的口腔检查进行了审查。这名5岁的孩子在服用丙戊酸钠18个月后出现了严重口腔炎。停药后口腔炎得到了缓解。文章还提供了相关文献的综述。口腔炎是丙戊酸钠治疗中罕见但潜在严重的不良反应。(《儿童神经病学杂志》2002年;17:225-227)。DOI:10.1177/088307380201700315
-
作为产物:描述:参考文献:名称:Bioavailable diacylhydrazine ligands for modulating the expression of exogenous genes via an ecdysone receptor complex摘要:本发明涉及用于核受体基于诱导基因表达系统的非甾体配体,以及一种调节外源基因表达的方法,其中包括一个包含:DNA结合结构域;配体结合结构域;转活化结构域;和配体的ecdysone受体复合物与包含外源基因和响应元件的DNA构建物接触;其中外源基因受响应元件控制,并且在配体存在的情况下DNA结合结构域与响应元件结合导致基因的激活或抑制。公开号:US20060020146A1
-
作为试剂:描述:2,2-二甲基-3-庚醇 、 、 在 正己烷 、 silica gel 、 silica 、 铬 、 2,2-二甲基-3-庚酮 、 dichloromethane hexane 、 ethyl acetate n-hexane 作用下, 以 二氯甲烷 为溶剂, 反应 24.0h, 以to yield 29.19 g of product at 83% yield的产率得到2,2-二甲基-3-庚酮参考文献:名称:Bioavailable diacylhydrazine ligands for modulating the expression of exogenous genes via an ecdysone receptor complex摘要:本发明涉及用于核受体基础诱导基因表达系统的非类固醇配体,以及一种调节外源基因表达的方法,其中包括接触含有以下内容的 DNA 结构:外源基因和响应元件,以及一个含有以下内容的蜕皮激素受体复合物:一个 DNA 结合域,一个配体结合域,一个转录激活域和一个配体。在配体的存在下,DNA 结合域与响应元件的结合导致基因的激活或抑制,而外源基因受响应元件控制。公开号:US09255273B2
文献信息
-
HIV protease inhibiting compounds申请人:Flentge Charles A.公开号:US20110003827A1公开(公告)日:2011-01-06A compound of the formula is disclosed as an HIV protease inhibitor. Methods and compositions for inhibiting an HIV infection are also disclosed.公开了一种具有以下公式的化合物,作为HIV蛋白酶抑制剂。还公开了抑制HIV感染的方法和组合物。
-
Reaction of lithium dialkylcuprates with S-2-pyridyl thioates in the presence of oxygen. A carboxylic ester synthesis
-
USES OF SESQUITERPENE LACTONE COMPOUNDS AND THEIR DERIVATIVES IN DRUGS PREPARATION申请人:ACCENDATECH公开号:US20160367525A1公开(公告)日:2016-12-22The present invention relates to the uses of sesquiterpene lactone compounds and their derivatives in preparing drugs. It belongs to the field of drug technology, specifically relates to the uses of the compounds of Formula (I) in preparing the drugs, especially the uses in preparing the drugs to treat rheumatoid arthritis and treat cancers through inhibiting cancer stem cells.
-
Organocopper reagents in dimethyl sulfide作者:Steven H. Bertz、Gary DabbaghDOI:10.1016/0040-4020(89)80070-5日期:1989.1Organocopper(I) reagents, RCu, are both more stable and more reactive when prepared in dimethyl sulfide instead of ether or tetrahydrofuran. A wide range of Li reagents has been investigated with good results, as has a selection of Grignard reagents. Excellent yields of products are observed with typical substrates such as α,β-unsaturated ketones and acid chlorides.
-
Reactivities of mixed organozinc and mixed organocopper reagents, 2. Selective n-alkyl transfer in tri-n-butylphosphine-catalyzed acylation of n-alkyl phenylzincs; an atom economic synthesis of n-alkyl aryl ketones作者:Ender Erdik、Özgen Ömür PekelDOI:10.1016/j.tetlet.2009.01.082日期:2009.4Tri-n-butylphosphine-catalyzed acylation of mixed n-alkyl phenylzincs with aromatic acyl halides in THF is efficient in selective transfer of n-alkyl groups to produce n-alkyl aryl ketones in good yields. This route provides an atom economic organocatalyzed alternative to transition metal-catalyzed acylation of di-n-alkylzincs.
表征谱图
-
氢谱1HNMR
-
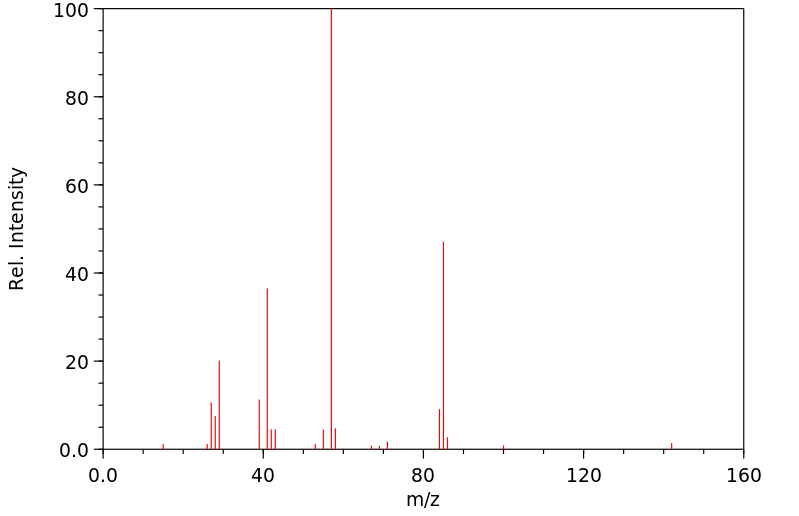
质谱MS
-
碳谱13CNMR
-
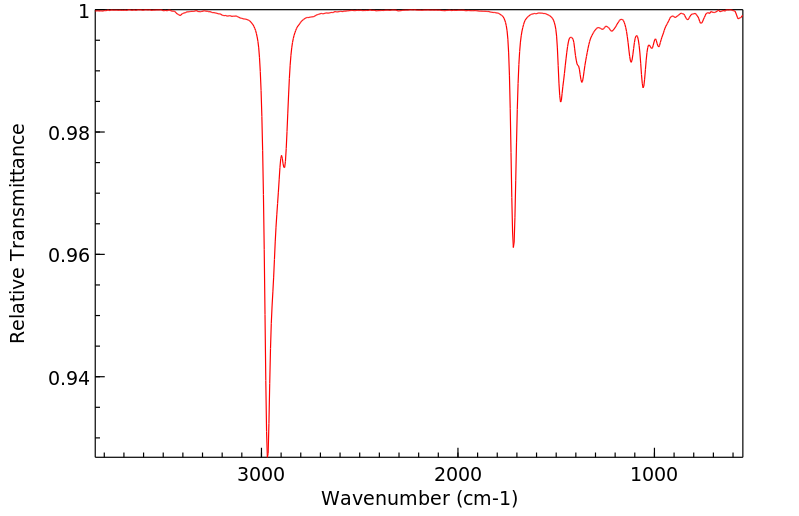
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷