邻苯二甲酸二烯丙酯 | 131-17-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-70 °C
-
沸点:165-167 °C/5 mmHg (lit.)
-
密度:1.121 g/mL at 25 °C (lit.)
-
蒸气密度:8.3 (vs air)
-
闪点:>230 °F
-
溶解度:0.18g/l
-
LogP:3.23 at 20℃
-
物理描述:Diallyl phthalate is a clear pale-yellow liquid. Odorless. (NTP, 1992)
-
颜色/状态:Nearly colorless, oily liquid
-
气味:mild lachrymatory
-
蒸汽密度:8.3 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1.16X10-3 mm Hg at 25 °C (est)
-
亨利常数:Henry's Law constant = 3.86X10-7 atm- cu m/mol at 25 °C (est)
-
自燃温度:385 °C
-
分解:When heated to decomp it emits acrid smoke and irritating fumes.
-
粘度:13 cP at 20 °C
-
聚合:Will polymerize if not inhibited; will polymerize with heat & catalyst
-
保留指数:1708 ;1718 ;1708 ;1698 ;1711 ;1712 ;1713 ;1713 ;1714 ;1714 ;1714 ;1692 ;1698 ;1712 ;1712
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:18
-
可旋转键数:8
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
TSCA:Yes
-
危险等级:9
-
危险品标志:Xn
-
安全说明:S24/25,S60,S61
-
危险类别码:R22,R50/53
-
WGK Germany:2
-
海关编码:29173400
-
危险品运输编号:UN 3082 9/PG 3
-
危险类别:9
-
RTECS号:CZ4200000
-
包装等级:III
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
制备方法与用途
邻苯二甲酸二异丙酯是一种反应型增塑剂,主要用于制备邻苯二甲酸二异丙酯树酯,并用作不饱和聚酯树脂的交联剂、纤维素树酯的增强剂以及在不加抑制剂时能自行聚合的树脂类的增塑剂。此外,还用于制备聚邻苯二甲酸二丙烯酯树脂,用作不饱和聚酯树脂的交联剂、乙烯基树脂的增塑剂、聚酯树脂的催化剂和颜料载体等。同时,也可作为许多聚合物及共聚物单体。
制法 酯化反应将计量的苯酐119kg、烯丙醇(加入1%的对苯二酚)121kg 和苯156kg 加入酯化釜中,逐步加入计量的硫酸(65%)。开始加热升温至80~95℃,反应过程中生成的水与苯共沸蒸出,冷凝液经苯水分离器分离除去水,苯流回反应釜直至每小时出水量<100ml结束反应并冷却。
中和洗涤将酯化反应生成物放入中和洗涤釜,补加苯68kg,加水200kg或液碱(5%)100kg中和。反复水洗中和,直至水层pH值为中性。
蒸馏脱苯洗涤中和好的物料放入脱苯蒸馏釜,以蒸汽加热,将苯蒸出,得到粗邻苯二甲酸二烯丙酯单体(DAP)。
真空精馏粗DAP单体在间歇蒸馏釜内,在温度180℃左右、压力266~532Pa(2~4mmHg)下进行精馏,收集180~200℃馏分为精DAP单体。
化学性质邻苯二甲酸二异丙酯是一种无色或淡黄色油状液体。气味温和但具有催泪性,凝固点-70℃,沸点158℃(0.53kPa),相对密度1.120(20/20℃),折射率1.520,闪点165.5℃,粘度13mPa·s。该物质不溶于水,但能溶于乙醇、乙醚、丙酮、苯等有机溶剂,在矿物油、甘油、乙二醇中部分溶解。
生产方法邻苯二甲酸酐与液碱反应生成邻苯二甲酸钠盐,再与氯丙烯在40-60℃、常压下酯化而得粗品。经过滤、中和、水洗、减压蒸馏后即为成品。原料消耗定额:苯酐(≥90%)780kg/t、氯丙烯(≥95)1060kg/t、液碱(40%)1080kg/t。
分类- 类别:易燃液体
- 毒性分级:中毒
-
急性毒性:
- 口服 - 大鼠 LD50: 656 毫克/ 公斤
-
刺激数据:
- 眼 - 兔子 500 毫克 轻度
- 爆炸物危险特性:本身无爆炸危险
- 可燃性危险特性:遇明火、高温、强氧化剂可燃;燃烧排放刺激烟雾
-
储运特性:
- 包装完整,轻装轻放;
- 库房通风,远离明火、高温,与氧化剂分开存放
泡沫、二氧化碳、干粉、砂土、雾状水
职业标准- TWA:5 毫克/ 立方米
- STEL:1 毫克/ 立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— o-((prop-2-enoxy)carbonyl)benzoic acid 3882-14-2 C11H10O4 206.198 —— 1-O-prop-2-enyl 2-O-prop-2-ynyl benzene-1,2-dicarboxylate 182342-88-7 C14H12O4 244.247 邻苯二甲酸二丁酯 Dibutyl phthalate 84-74-2 C16H22O4 278.348 邻苯二甲酸 benzene-1,2-dicarboxylic acid 88-99-3 C8H6O4 166.133 苯酐 phthalic anhydride 85-44-9 C8H4O3 148.118 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (4Z)-3,6-二氢-2,7-苯并二氧杂环癸烷e-1,8-二酮 3,6-Dihydro-2,7-benzodioxecine-1,8-dione 406911-89-5 C12H10O4 218.209 —— (5E,19E)-3,8,17,22-tetraoxatricyclo[22.4.0.010,15]octacosa-1(28),5,10,12,14,19,24,26-octaene-2,9,16,23-tetrone 1608129-94-7 C24H20O8 436.418 3,4,5,6-四氢-2,7-苯并二氧杂环癸烷e-1,8-二酮 3,4,5,6-tetrahydrobenzo[c][1,6]dioxecine-1,8-dione 29246-20-6 C12H12O4 220.225 邻苯二甲酸 benzene-1,2-dicarboxylic acid 88-99-3 C8H6O4 166.133 —— Tetrachlor-phthalsaeure-diallylester 3488-10-6 C14H10Cl4O4 384.043
反应信息
-
作为反应物:参考文献:名称:Process for the manufacture of organic compounds摘要:本发明涉及一种用于制造血管紧张素受体拮抗剂(ARB:也称为血管紧张素II受体拮抗剂或AT1受体拮抗剂)及其盐的工艺,涉及新颖的中间体和工艺步骤。公开号:EP2316821A1
-
作为产物:描述:1-O-prop-2-enyl 2-O-prop-2-ynyl benzene-1,2-dicarboxylate 在 indium 作用下, 以 乙醇 为溶剂, 反应 40.0h, 以95%的产率得到邻苯二甲酸二烯丙酯玻璃纤维增强塑料参考文献:名称:Selective Reduction of Terminal Alkynes to Alkenes by Indium Metal摘要:DOI:10.1021/jo010262z
-
作为试剂:描述:聚丁二酸乙二醇酯(DEGS) 在 Trititanium oxide poly(diallylisophthalate) 、 ethylene-vinyl 、 alcohol 、 乙酸乙烯酯 作用下, 以 二氯甲烷 、 邻苯二甲酸二烯丙酯玻璃纤维增强塑料 、 丙酮 、 cellulose propionate 为溶剂, 生成 乙烯 、 乙烯醇 、 、 乙酸乙烯酯参考文献:名称:Coated paper containing a plastic supporting substrate摘要:一种不易撕裂的涂层纸,包括塑料支撑基质、粘合剂层和颜料层。粘合剂层由以下聚合物中选出:(1)羟丙基纤维素,(2)聚乙烯基烷醚,(3)吡咯烷酮/醋酸乙烯酯,(4)季铵化吡咯烷酮/二烷基烷基氨基乙基/甲基丙烯酸酯,(5)聚乙烯吡咯烷酮,(6)聚乙烯亚胺和它们的混合物组成;并且具有墨水吸收聚合物层。公开号:US05075153A1
文献信息
-
AQUEOUS CURABLE COMPOSITION AND WATER-SOLUBLE PHOTOPOLYMERIZATION INITIATOR申请人:FUJIFILM CORPORATION公开号:US20180362558A1公开(公告)日:2018-12-20An aqueous curable composition includes a water-soluble photopolymerization initiator having a structure in which one or more carbonyl groups further directly bond to an aromatic ring of an aromatic acyl group that bonds to a phosphorus atom in an acylphosphine oxide structure, water, and a polymerizable compound. A novel water-soluble photopolymerization initiator is a compound represented by formula 1-1 or formula 2-1.
-
[EN] POLYCATIONIC AMPHIPHILES AND POLYMERS THEREOF AS ANTIMICROBIAL AGENTS AND METHODS USING SAME<br/>[FR] COMPOSÉS AMPHIPHILES POLYCATIONIQUES ET LEURS POLYMÈRES UTILISABLES EN TANT QU'AGENTS ANTIMICROBIENS ET LEURS PROCÉDÉS D'UTILISATION申请人:TEMPLE UNIVERSITY-OF THE COMMONWEALTH SYSTEM OF HIGHER EDUCATION公开号:WO2016172436A1公开(公告)日:2016-10-27The present invention includes novel polycationic amphiphilic compounds useful as antimicrobial agents. The present invention further includes novel polymers of polycationic amphiphilic compounds useful as antimicrobial agents. The present invention further includes methods useful for removing microorganisms and/or biofilm-embedded microorganisms from a surface. The present invention further includes compositions and methods useful for preventing or reducing the growth or proliferation of microorganisms and/or biofilm-embedded microorganisms on a surface.
-
[EN] PROCESS FOR THE MANUFACTURING OF A POLYMER WITH URETHANE GROUPS<br/>[FR] PROCÉDÉ DE FABRICATION D'UN POLYMÈRE AYANT DES GROUPES URÉTHANNE申请人:BASF SE公开号:WO2019034473A1公开(公告)日:2019-02-21Process for the manufacturing of a polymer with urethane groups, wherein in a first alternative a compound A) with at least two five-membered cyclic monothiocarbonate groups and a compound B) with at least two amino groups, selected from primary or secondary amino groups and optionally a compound C) with at least one functional group that reacts with a group -SH are reacted or wherein in a second alternative a compound A) with at least two five-membered cyclic monothiocarbonate groups or a mixture of a compound A) with a compound A1)with one five-membered cyclic monothiocarbonate group and a compound B) with at least two amino groups, selected from primary or secondary amino groups or a compound B1) with one amino group selected from primary or secondary amino groups or mixtures of compounds B) and B1) and a compound C) with at least two functional groups that react with a group -SH or in case of a carbon-carbon triple bond as functional group that react with a group -SH, a compound C) with at least one carbon-carbon triple bond. are reacted.制造含有尿烷基团的聚合物的过程,其中,在第一种替代方案中,至少具有两个五元环状单硫代碳酸酯基团的化合物A)与至少具有两个氨基的化合物B)反应,这些氨基选自伯氨基或仲氨基,以及可选的至少含有一个与-SH基团反应的功能性基团的化合物C);或者在第二种替代方案中,至少具有两个五元环状单硫代碳酸酯基团的化合物A)或化合物A)与具有一个五元环状单硫代碳酸酯基团的化合物A1)的混合物与至少具有两个氨基的化合物B)反应,这些氨基选自伯氨基或仲氨基,或具有一个氨基的化合物B1),该氨基选自伯氨基或仲氨基,或化合物B)和B1)的混合物,以及至少具有两个与-SH基团反应的功能性基团的化合物C),或当功能性基团为碳-碳三键时,至少具有一个碳-碳三键的化合物C)。
-
DENTAL COMPOSITIONS COMPRISING ADDITION-FRAGMENTATION AGENTS申请人:Joly Guy D.公开号:US20160008234A1公开(公告)日:2016-01-14A curable dental composition comprising an addition-fragmentation agent and a curable dental resin is disclosed.揭示了一种可治愈的牙科组合物,包括一种加成-断裂剂和一种可治愈的牙科树脂。
-
Thermotropic liquid crystal polymer microcapsules, a method for preparing the same, and cosmetic compositions containing the same申请人:——公开号:US20030129247A1公开(公告)日:2003-07-10There are provided thermotropic liquid crystal polymer microcapsules which can show behavior of liquid crystal as it is within polymer phase due to phase separation between liquid crystal and polymer, so to be incorporated into cosmetic composition as an additive for visual effect, and in loading active ingredients within liquid crystal, can improve the stability of the active ingredients in cosmetic base; and a method for preparing the same; and cosmetic compositions containing the same.提供了热致液晶聚合物微胶囊,由于液晶与聚合物之间的相分离,这些微胶囊可以展现液晶在聚合物相中的行为,因此可以作为化妆品组合物中的添加剂用于视觉效果,并且在液晶中装载活性成分,可以提高这些活性成分在化妆品基料中的稳定性;以及制备这些微胶囊的方法;以及含有这些微胶囊的化妆品组合物。
表征谱图
-
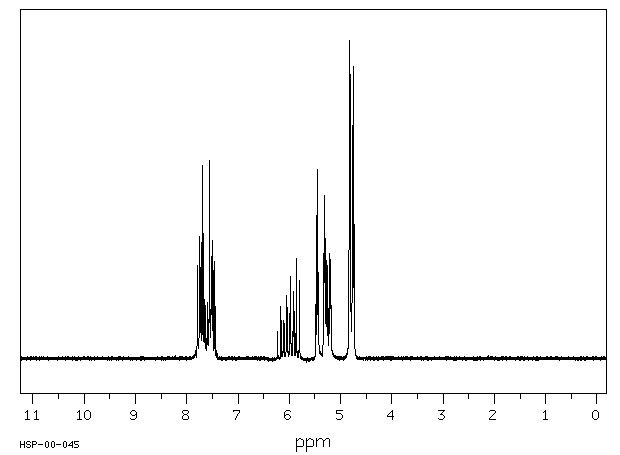
氢谱1HNMR
-
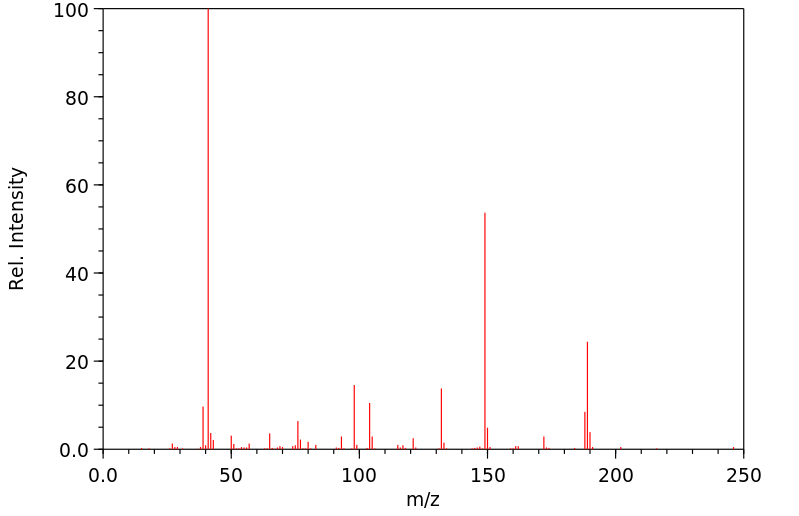
质谱MS
-
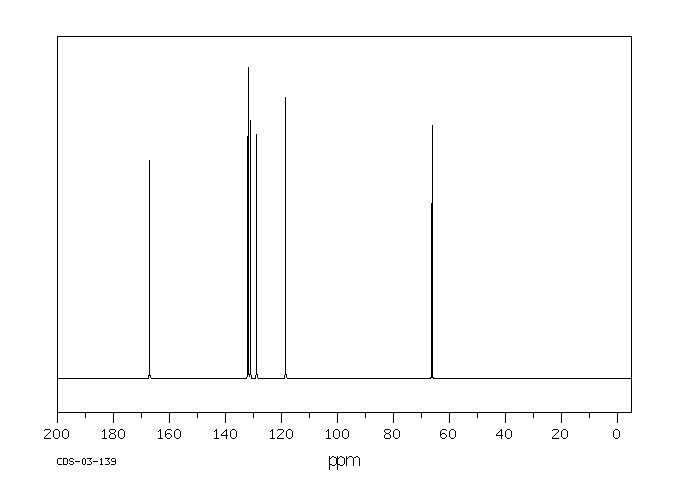
碳谱13CNMR
-
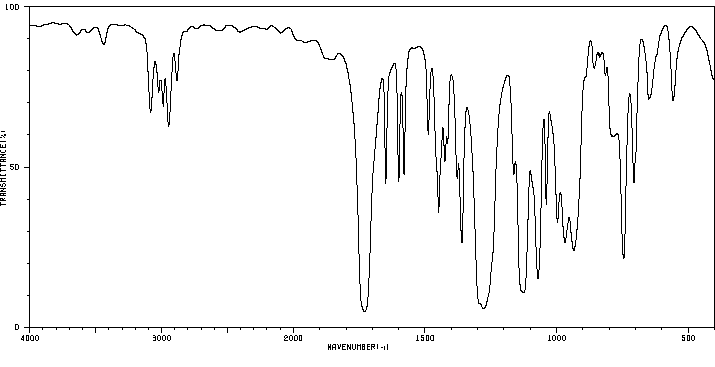
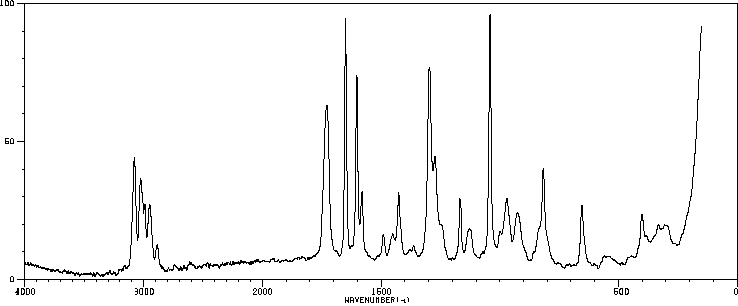
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息