N-乙基-p-甲氧基-alpha-甲基苯乙胺 | 14367-46-5
中文名称
N-乙基-p-甲氧基-alpha-甲基苯乙胺
中文别名
N-乙基-4-甲氧基-α-甲基-苯乙胺;N-乙基-1-(4-甲氧基苯基)丙-2-胺;N-乙基-1-(4-甲氧基苯基)丙基-2-胺;N-乙基-p-甲氧基-α-甲基苯乙胺
英文名称
N-ethyl-p-methoxy-a-methyl-Phenethylamine
英文别名
ethyl-[2-(4-methoxy-phenyl)-1-methyl-ethyl]-amine;Aethyl-[2-(4-methoxy-phenyl)-1-methyl-aethyl]-amin;2-Aethylamino-1-(4-methoxy-phenyl)-propan;N-ethyl-p-methoxy-α-methylphenethylamine;N-ethyl-[1-methyl-2-(4-methoxyphenyl)]-ethylamine;N-ethyl-3-(4-methoxyphenyl)-2-propylamine;N-Ethyl-p-methoxy-alpha-methylphenethylamine;N-ethyl-1-(4-methoxyphenyl)propan-2-amine
CAS
14367-46-5
化学式
C12H19NO
mdl
MFCD00236007
分子量
193.289
InChiKey
USBWBBAUWVUJLA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:269.89℃[at 101 325 Pa]
-
密度:0.943[at 20℃]
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:2.6-2.79 at 25℃
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:14
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:21.3
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(4-甲氧基苄基)乙胺 2-amino-1-(4-methyoxyphenyl)propane 64-13-1 C10H15NO 165.235 对甲氧基苯基丙酮 4-methoxybenzyl methyl ketone 122-84-9 C10H12O2 164.204 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二乙基-1-甲基-2-(对甲氧基苯基)乙胺 N,N-diethyl-1-methyl-2-(p-methoxyphenyl)ethylamine 195213-66-2 C14H23NO 221.343 —— 4-Hydroxy-Phenethylamine 69389-96-4 C11H17NO 179.262 —— 5-[n-ethyl-[1-methyl-2-(4-methoxyphenyl)]-ethylamino]-1-pentane carboxylic acid ethylester 57646-55-6 C20H33NO3 335.487
反应信息
-
作为反应物:描述:N-乙基-p-甲氧基-alpha-甲基苯乙胺 在 CYP2D6 作用下, 以 phosphate buffer 为溶剂, 反应 2.0h, 生成 4-Hydroxy-Phenethylamine参考文献:名称:Involvement of CYP2D6 in the in vitro metabolism of amphetamine, two N-alkylamphetamines and their 4-methoxylated derivatives摘要:1. Amphetamine (AM) and five amphetamine derivatives, N-ethylamphetamine (NEA), N-butylamphetamine (NBA), 4-methoxyamphetamine (RI-AM), 4-methoxy-N-ethylamphetamine (M-NEA) and 4-methoxy-N-butylamphetamine (M-NBA) were incubated with microsomal preparations from cells expressing human CYP2D6 to determine whether the enzyme was capable of catalyzing the direct ring oxidation of all substrates; the N-dealkylation of NEA, NEA, M-NEA and M-NBA; and the O-demethylation of M-AM, M-NEA and M-NBA.2. None of the six compounds examined was N-dealkylated to any extent.3. The only metabolites produced from,AM, NEA and NEA were the corresponding ring 4-hydroxylated compounds, and the rates of formation were low.4. All ring l-methoxylated substrates were efficiently O-demethylated by CYP2D6 to their corresponding phenols. The size of the N-alkyl group influenced the rates of formation of these phenolamines. In contrast to reported findings with 2- and 3-methoxyamphetamines, none of the 4-methoxyamphetamines was ring-oxidized in the CYP2D6 enzyme system to 2- or 3-hydroxy-4-methoxyamphetamines or to dihydroxy-amphetamines.DOI:10.1080/004982599238344
-
作为产物:描述:参考文献:名称:Fusco; Caggianelli, Il Farmaco, scienza e tecnica, 1948, vol. 3, p. 125,133摘要:DOI:
文献信息
-
AMPHETAMINE CONTROLLED RELEASE, PRODRUG, AND ABUSE-DETERRENT DOSAGE FORMS申请人:CHEMAPOTHECA, LLC公开号:US20180243241A1公开(公告)日:2018-08-30The invention also relates to pharmaceutical compositions comprising highly pure amphetamine and amphetamine-class compounds resulting from the synthesis of chiral and racemic amphetamine derivatives by stereospecific, regioselective cuprate addition reaction with aziridine phosphoramidate compounds, and to methods of manufacturing, delivering, and using the amphetamine compounds resulting from the synthesis of chiral and racemic amphetamine derivatives by stereospecific, regioselective cuprate addition reaction with aziridine phosphoramidate compounds.
-
[EN] AMPHETAMINE CONTROLLED RELEASE, PRODRUG, AND ABUSE DETERRENT DOSAGE FORMS<br/>[FR] LIBÉRATION CONTRÔLÉE DE L'AMPHÉTAMINE, PROMÉDICAMENT ET FORMES POSOLOGIQUES DISSUASIVES DU MÉSUSAGE申请人:CHEMAPOTHECA LLC公开号:WO2017147375A1公开(公告)日:2017-08-31The invention also relates to pharmaceutical compositions comprising highly pure amphetamine and amphetamine-class compounds resulting from the synthesis of chiral and racemic amphetamine derivatives by stereospecific, regioselective cuprate addition reaction with aziridine phosphoramidate compounds, and to methods of manufacturing, delivering, and using the amphetamine compounds resulting from the synthesis of chiral and racemic amphetamine derivatives by stereospecific, regioselective cuprate addition reaction with aziridine phosphoramidate compounds.
-
[EN] AMIDE LINKER PEROXISOME PROLIFERATOR ACTIVATED RECEPTOR MODULATORS<br/>[FR] MODULATEURS DE RECEPTEUR ACTIVE DE LA PROLIFERATION DES PEROXISOMES A LIEUR AMIDE申请人:LILLY CO ELI公开号:WO2004000789A1公开(公告)日:2003-12-31The present invention is directed to compounds, compositions, and use of compounds the structural Formula (I).本发明涉及结构式(I)的化合物、组合物及其使用。
-
Ind2TiMe2-Catalyzed Addition of Methyl- and Ethylamine to Alkynes作者:Klaudia Marcseková、Bernd Wegener、Sven DoyeDOI:10.1002/ejoc.200500458日期:2005.11simple hydrogenation-like experimental protocol for the addition of gaseous methyl- and ethylamine to alkynes in the presence of Ind2TiMe2 as the catalyst. For efficient hydroamination reactions it is sufficient to stir a mixture of the alkyne and the catalyst in toluene at temperatures between 80 °C (terminal alkynes) and 105 °C (internal alkynes) under a constant pressure of 1 atm of the corresponding
-
Aralkylamino sulfones having spasmolytic activities
表征谱图
-
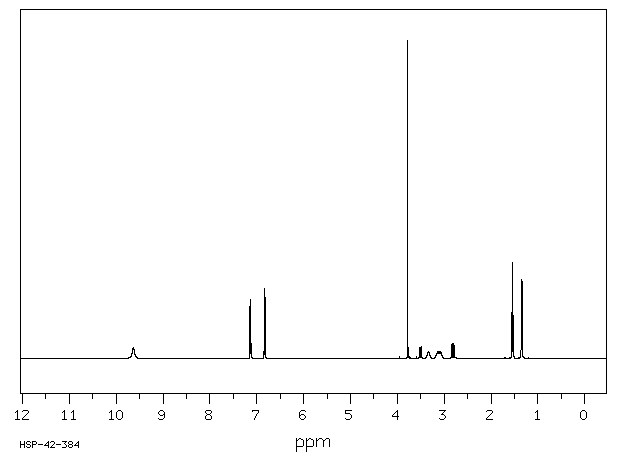
氢谱1HNMR
-
质谱MS
-
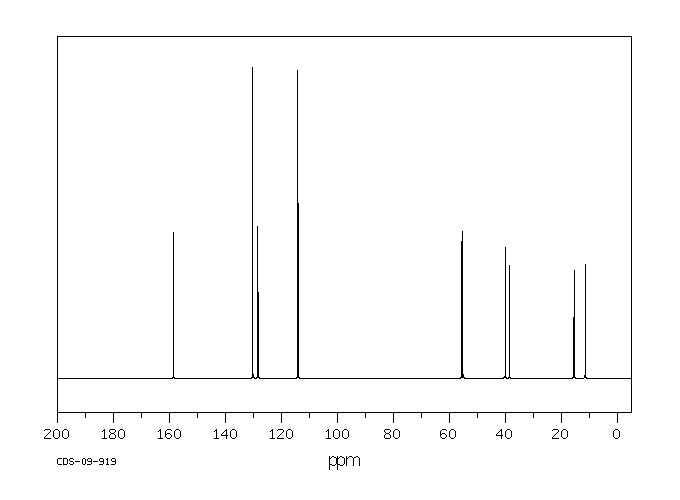
碳谱13CNMR
-
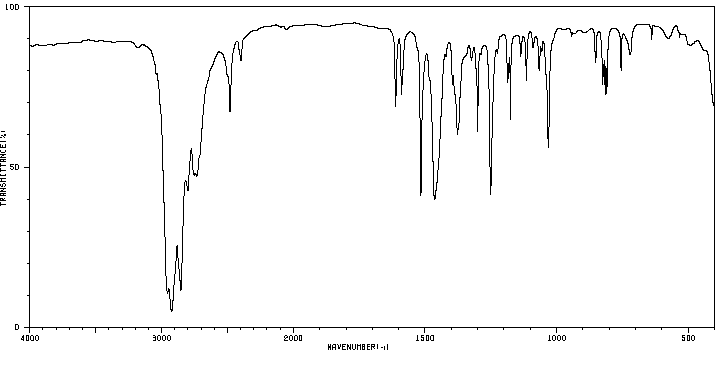
红外IR
-
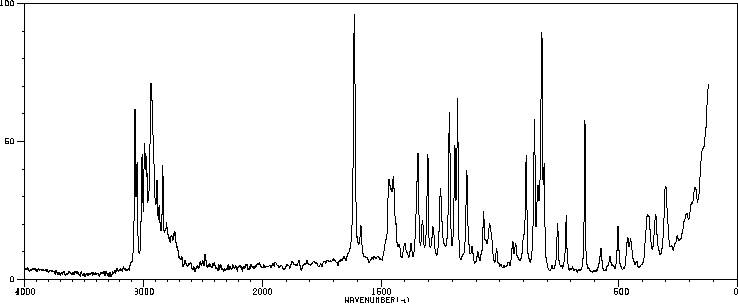
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫