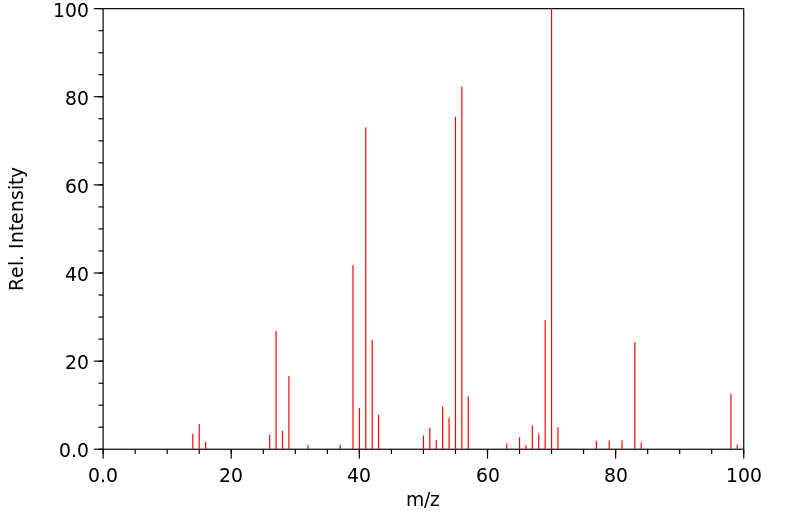
Present information on the spatial configuration of the dialkyl cyclopentanes and cyclohexanes is unsatisfactory. Zelinsky1 concluded from the result of an asymmetric synthesis that the lower-boiling isomer of 1: 3-dimethylcyclohexane had the trans configuration, and following on this later workers have assigned the trans structure to the lower boiling isomers of naphthenes. Mousseron and Granger2 carried out a more reliable asymmetric synthesis of 1: 3-dimethyl-cyclohexane and showed that the higher-boiling isomer was optically active and therefore of trans configuration. Chiurdoglu3 afterwards compared the relative rates of oxidation and bromination of the spatial isomers of various 1: 2-dialkylcyclopentanes, and found that the higher-boiling isomers possessed the greater reactivity. It was also found that trans l: 3-dimethylcyclohexane, as determined by Mousseron and Granger (loc. cit), and trans decalane reacted more rapidly with the above reagents than did their cis isomers. Chiurdoglu, therefore, concluded that the higher-boiling isomers of the 1: 2-dialkylcyclopentanes had the trans configuration. Pitzer and Beckett4, in a recent investigation of the theoretical and calculated entropies of the dimethylcyclohexanes, concluded that the nomenclature of the 1: 3-dimethylcyclohexanes should be reversed and that the higher-boiling isomer should be regarded as the trans isomer. This result is thus in agreement with that obtained by Mousseron and Granger (loc. cit.).
目前关于二烷基
环戊烷和
环己烷的空间构型的信息并不令人满意。Zelinsky1根据不对称合成的结果得出结论,1:3-二
甲基环己烷的低沸点异构体具有反式构型,后来,研究人员将环烷的低沸点异构体也归为反式结构。Mousseron和Granger2对1:3-二
甲基环己烷进行了更可靠的不对称合成,并发现高沸点异构体具有光学活性,因此具有反式构型。之后,Chiurdoglu3比较了各种1:2-二烷基
环戊烷的空间异构体的氧化和
溴化反应的相对速率,发现高沸点异构体的反应性更强。他们还发现,反式l:3-二
甲基环己烷(由Mousseron和Granger确定,同上)和反式
癸烷与上述试剂的反应速度比顺式异构体更快。因此,Chiurdoglu得出结论,1:2-二烷基
环戊烷的高沸点异构体具有反式构型。Pitzer和Beckett4在最近对二
甲基环己烷的理论熵和计算熵的研究中得出结论,1:3-二
甲基环己烷的命名应颠倒过来,高沸点异构体应被视为反式异构体。因此,这一结果