双(2-氯苯基)磷酰氯 | 17776-78-2
中文名称
双(2-氯苯基)磷酰氯
中文别名
——
英文名称
bis(2-chlorophenyl) chlorophosphate
英文别名
bis(2-chlorophenyl)chlorophosphate;chlorophosphoric acid bis-(2-chloro-phenyl ester);Chlorophosphorsaeure-bis-(2-chlor-phenylester);Bis(2-chlorophenyl) phosphorochloridate;1-chloro-2-[chloro-(2-chlorophenoxy)phosphoryl]oxybenzene
CAS
17776-78-2
化学式
C12H8Cl3O3P
mdl
MFCD00015736
分子量
337.526
InChiKey
ZLSGEMKKBUVQOM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:8
-
危险类别码:R34
-
危险品运输编号:UN 3265
-
包装等级:III
-
危险类别:8
-
安全说明:S26,S36/37/39,S45
-
储存条件:常温下应密闭、避光保存,并放置于通风、干燥的环境中,在惰性气体保护下更为理想。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三(2-氯苯基)亚磷酸酯 tri-(2-chlorophenyl) phosphite 24460-31-9 C18H12Cl3O3P 413.624 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— phosphoric acid bis-(2-chloro-phenyl ester) 36400-49-4 C12H9Cl2O4P 319.081 —— bis(2-chlorophenyl)amidophosphate 116992-74-6 C12H10Cl2NO3P 318.096 丁基双(2-氯苯基)磷酸酯 butyl bis(2-chlorophenyl) phosphate 1021947-60-3 C16H17Cl2O4P 375.188
反应信息
-
作为反应物:描述:双(2-氯苯基)磷酰氯 在 水 作用下, 反应 13.0h, 以59%的产率得到phosphoric acid bis-(2-chloro-phenyl ester)参考文献:名称:Hydrogen phosphates: Self initiated organocatalysts for the controlled ring-opening polymerization of cyclic esters摘要:A series of arylhydrogenphosphates and aryldihydrogenphosphates was synthesized and characterized using spectroscopic methods and single crystal X-ray diffraction. These compounds were assessed as catalysts towards the ring-opening polymerization and proved to be potent organocatalysts for the ring-opening polymerization of cyclic esters. The bulk polymerizations were performed in the absence of external initiator. The polymerization proceeds in a controlled fashion which leads to well defined polyesters with narrow molecular weight distributions. In the post polymerization experiments, kinetics, mechanism and monomer concentration effects were investigated. The kinetic results have confirmed the pseudo-living character of the polymerizations and mechanistic studies suggest that the polymerization operates through a cationic mechanism. (C) 2013 Elsevier B.V. All rights reserved.DOI:10.1016/j.ica.2013.02.006
-
作为产物:描述:参考文献:名称:Hydrogen phosphates: Self initiated organocatalysts for the controlled ring-opening polymerization of cyclic esters摘要:A series of arylhydrogenphosphates and aryldihydrogenphosphates was synthesized and characterized using spectroscopic methods and single crystal X-ray diffraction. These compounds were assessed as catalysts towards the ring-opening polymerization and proved to be potent organocatalysts for the ring-opening polymerization of cyclic esters. The bulk polymerizations were performed in the absence of external initiator. The polymerization proceeds in a controlled fashion which leads to well defined polyesters with narrow molecular weight distributions. In the post polymerization experiments, kinetics, mechanism and monomer concentration effects were investigated. The kinetic results have confirmed the pseudo-living character of the polymerizations and mechanistic studies suggest that the polymerization operates through a cationic mechanism. (C) 2013 Elsevier B.V. All rights reserved.DOI:10.1016/j.ica.2013.02.006
-
作为试剂:描述:N4-苯甲酰基-5′-O-(4,4′-二甲氧基三苯基)-2′-脱氧胞苷 在 吡啶 、 盐酸 、 4-二甲氨基吡啶 、 双(2-氯苯基)磷酰氯 、 三乙胺 作用下, 以 1,4-二氧六环 、 二氯甲烷 为溶剂, 反应 2.49h, 生成 [(2R,3S,5R)-5-(4-benzamido-2-oxopyrimidin-1-yl)-2-[[[(2R,3S,5R)-5-(6-benzamidopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl]oxy-(4-chlorophenyl)sulfanylphosphoryl]oxymethyl]oxolan-3-yl] 4-oxopentanoate参考文献:名称:Reese, Colin B.; Quanlai, Song, Journal of the Chemical Society. Perkin transactions I, 1999, # 11, p. 1477 - 1486摘要:DOI:
文献信息
-
[EN] TRI-FUNCTIONAL CROSSLINKING REAGENTS<br/>[FR] RÉACTIFS DE RÉTICULATION TRIFONCTIONNELS申请人:ETH ZUERICH公开号:WO2017081069A1公开(公告)日:2017-05-18The present invention relates to tri-functional crosslinking reagents carrying (i) a ligand-reactive group for conjugation to a ligand of interest having at least one binding site on a target glycoprotein receptor, (ii) a hydrazone group for the capturing of oxidized receptor-glycoproteins and (iii) an affinity group selected from azides and aklynes for the detection, isolation and purification of captured glycoproteins; as well as their manufacturing. The invention further provides for improved methods of detecting, identifying and characterizing interactions between ligands and their corresponding target glycoproteins on living cells and in biological fluids. The invention further provides for new uses of catalysts in such methods.
-
Nucleotide, XVIII. Synthese und Eigenschaften von (tert-Butyldimethylsilyl)guanosinen, Guanosin-3?-phosphotriestern und Guanosin-haltigen Oligoncleotiden作者:Dieter Flockerzi、Wilhelm Schlosser、Wolfgang PfleidererDOI:10.1002/hlca.19830660718日期:1983.11.2Nucleotides, XVIII. Synthesis and Properties of (tert-Butyldimethylsilyl)guanosines, Guanosine-3′-Phosphotriesters and Guanosine-containing Oligonucleotides
-
Butyrylcholinesterase Inhibitors申请人:JAL Therapeutics, LLC公开号:US20160206635A1公开(公告)日:2016-07-21Butyrylcholinesterase inhibitors, their formulation, and their use primarily in the treatment of neurodegenerative diseases. These inhibitors generally are phosphates, phosphonates, phosphinates, and phosphoramidates. These inhibitors can be incorporated in pharmaceutical compositions and administered to a patient in therapeutically effective amounts to treat neurodegenerative diseases.
-
2-5A ANALOGS AND THEIR METHODS OF USE申请人:Beigelman Leonid公开号:US20100331397A1公开(公告)日:2010-12-30Disclosed herein are compounds that activate RNaseL, methods of synthesizing compounds that activate RNaseL and the use of compounds that activate RNaseL for treating and/or ameliorating a disease or a condition, such as a viral infection, a bacterial infection, cancer and/or parasitic disease.本文披露了激活RNaseL的化合物,合成激活RNaseL的化合物的方法,以及利用激活RNaseL的化合物治疗和/或改善疾病或状况的用途,例如病毒感染、细菌感染、癌症和/或寄生虫病。
-
BUTYRYLCHOLINESTERASE INHIBITORS申请人:Acey Roger A.公开号:US20100069337A1公开(公告)日:2010-03-18Butyrylcholinesterase inhibitors, their formulation, and their use primarily in the treatment of neurodegenerative diseases. These inhibitors generally are phosphates, phosphonates, phosphinates, and phosphoramidates. These inhibitors can be incorporated in pharmaceutical compositions and administered to a patient in therapeutically effective amounts to treat neurodegenerative diseases.
表征谱图
-
氢谱1HNMR
-
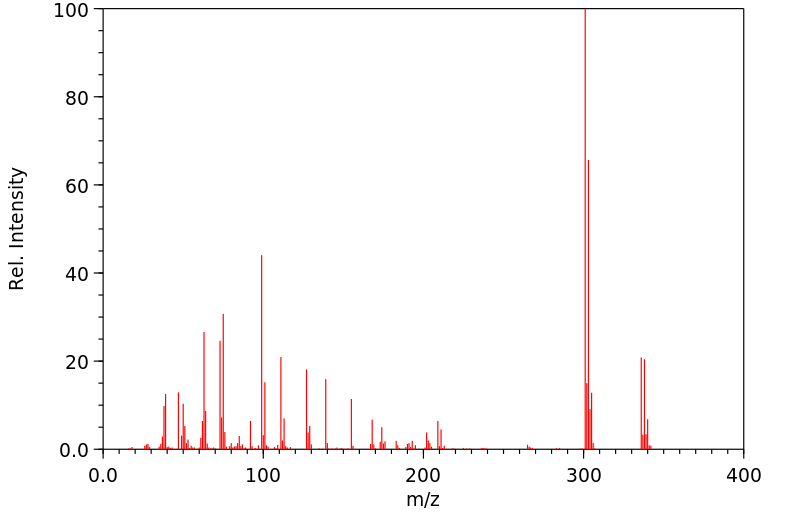
质谱MS
-
碳谱13CNMR
-
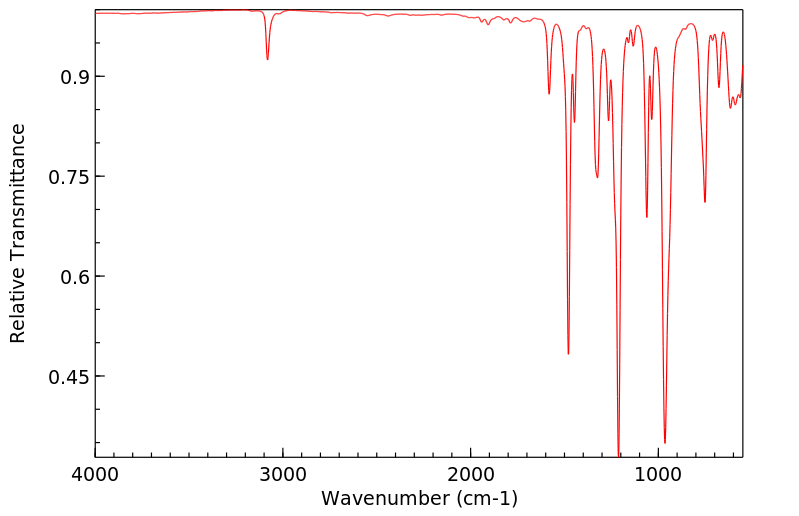
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫