1-环己基-1-戊醇 | 63126-49-8
中文名称
1-环己基-1-戊醇
中文别名
(1-羟基戊基)环己烷
英文名称
(+/-)-1-cyclohexyl-1-pentanol
英文别名
1-cyclohexyl-1-pentanol;1-cyclohexylpentanol;1-cyclohexylpentan-1-ol
CAS
63126-49-8;93548-33-5;114376-32-8;7338-43-4
化学式
C11H22O
mdl
——
分子量
170.295
InChiKey
PKXSPCMDZCKLCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:0.90
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
反应信息
-
作为反应物:描述:参考文献:名称:Empirical method for predicting enantioselectivity in catalytic reactions: demonstration with lipase and oxazaborolidine摘要:We derived a novel equation capable of predicting the degree of enantioselectivity in a catalytic reaction without any knowledge of the reaction mechanism and/or the transition-state structure. and tested the validity of this equation by changing substrates systematically in the lipase or oxazaborolidine-catalyzed reactions A good correlation was observed between the predicted and observed E values, and the stereochemistry of the products Could be predicted correctly in most cases (28 out of 30) (C) 2009 Elsevier Ltd All rights reservedDOI:10.1016/j.tet.2009.09.058
-
作为产物:描述:参考文献:名称:从醇的合成米-Fluorophenylsulfones和Dialkylboranes:应用E7389的C14-C35大厦摘要:的反应米-fluorophenylsulfone阴离子与dialkylboranes,随后碱性过氧化氢的氧化,以高收率收率醇。据报道,该方法的工艺,范围和局限性得到优化,并被用于合成E7389的一种C14–C35构建基块,这是葫芦素B的右半类似物。DOI:10.1021/ol300672q
文献信息
-
A new type of complex reagent, R4Pb / TiCl4作者:Yoshinori Yamamoto、Jun-ichi Yamada、Tetsuya AsanoDOI:10.1016/0040-4020(92)80009-5日期:1992.7Tetraalkllleads (R4Pb) reacted quite smoothly with aldehydes R′CHO in the presence of TiCl4 to produce the corresponding alcohols (RCHOHR′) in high to good yields. The reagent system, R4Pb/TiCl4, exhibited high chemoselectivity; only aldehydes underwent the alkylation in the presence of ketones. Further, the new reagent exhibited high 1,2- and 1,3-asymmetric induction. The transfer order of alkyl groups
-
A new convenient procedure to prepare organomanganese reagents from organic halides and activated manganese作者:Gérard Cahiez、Arnaud Martin、Thomas DelacroixDOI:10.1016/s0040-4039(99)01331-3日期:1999.8A new method to obtain activated manganese metal, especially attractive for large scale preparative organic chemistry, is described. The key point is the use of 2-phenylpyridine as electron carrier to reduce manganese chloride by lithium. The active manganese thus obtained was used to prepare various organomanganese reagents from organic halides. The reactivity of these reagents has been studied (acylation
-
Pentamethylcyclopentadienide in organic synthesis: nucleophilic addition of lithium pentamethylcyclopentadienide to carbonyl compounds and carbon–carbon bond cleavage of the adducts yielding the parent carbonyl compounds作者:Minoru Uemura、Kazunari Yagi、Masayuki Iwasaki、Kenichi Nomura、Hideki Yorimitsu、Koichiro OshimaDOI:10.1016/j.tet.2006.01.097日期:2006.4dienide (C5Me5Li, Cp*Li) reacted with aromatic aldehyde to provide the corresponding carbinol in excellent yield. The carbinol returns to the parent aldehyde and pentamethylcyclopentadiene upon exposure to acid or due to heating. Chlorodimethylaluminum is essential as an additive to attain the nucleophilic addition of Cp*Li to aliphatic aldehyde. The carbinol derived from aliphatic aldehyde returns
-
Selectivity in Organic Group Transfer in Reactions of Mixed Diorganomanganese(II) and Triorganomanganate(II) with 2-Cyclohexen-1-one or Cyclohexanecarbaldehyde作者:Hideki Yorimitsu、Yasuhiro Hayashi、Jun Tang、Hiroshi Shinokubo、Koichiro OshimaDOI:10.1246/bcsj.70.2297日期:1997.9The unsymmetrical diorganomanganeses(II) (R1R2Mn) and magnesium triorganomanganates(II) (R12R2MnMgX) reacted with 2-cyclohexen-1-one to produce the 1,4-addition products in moderate to good yields. The approximate reactivity order obtained from the product distribution was CH2=CHCH2 > PhS > n-Bu > Ph > Me, Me3SiCH2, n-C6H13–C≡C. In contrast, the reactivity order for the addition of these reagents to
-
Nickel-Catalyzed Alkylation of Aldehydes with Trialkylboranes作者:Koji Hirano、Hideki Yorimitsu、Koichiro OshimaDOI:10.1021/ol051917b日期:2005.10.1[reaction: see text] Nickel-catalyzed alkylation of aldehydes with trialkylboranes proceeds smoothly in the presence of a catalytic amount of 5-allyl-1,2,3,4,5-pentamethyl-1,3-cyclopentadiene or an excess of cesium carbonate to afford the corresponding secondary alcohols.
表征谱图
-
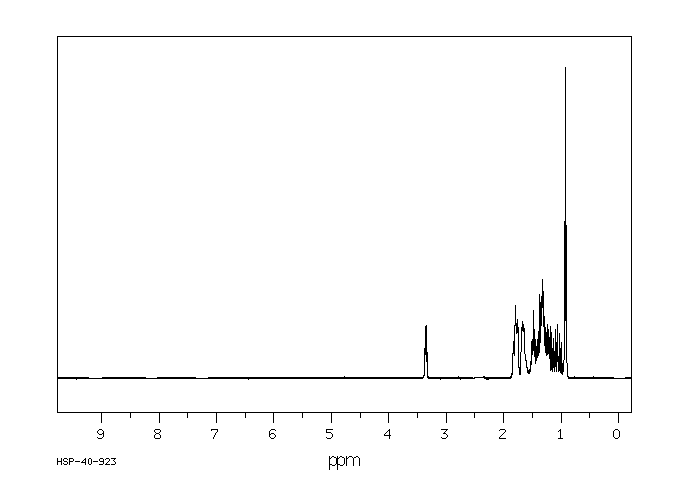
氢谱1HNMR
-
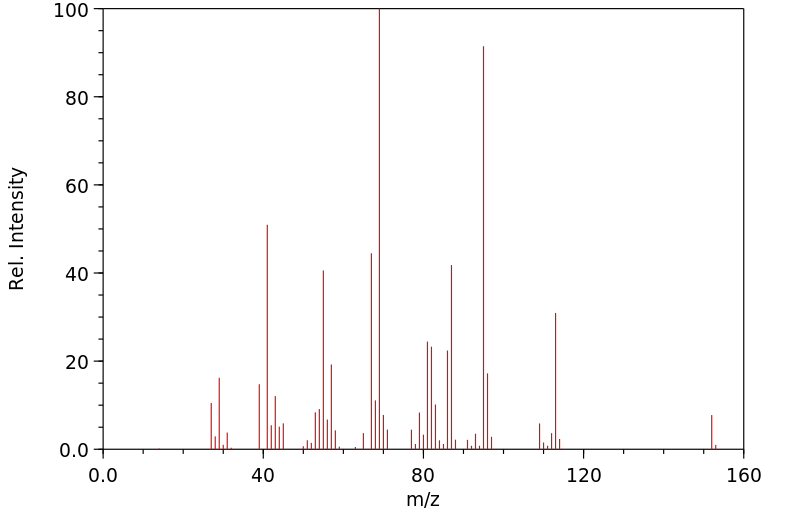
质谱MS
-
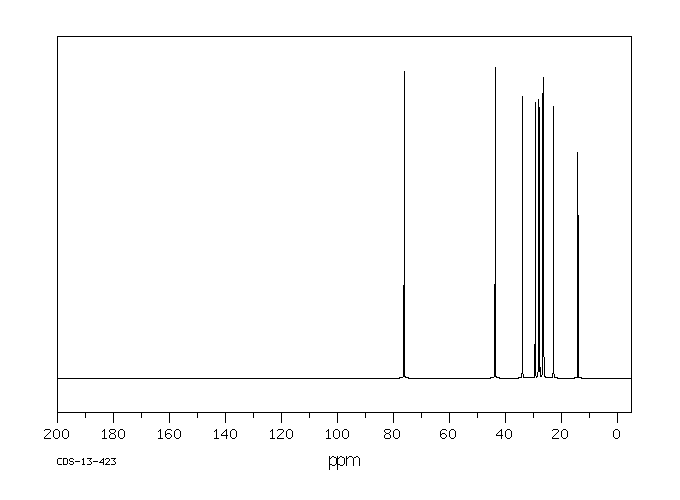
碳谱13CNMR
-
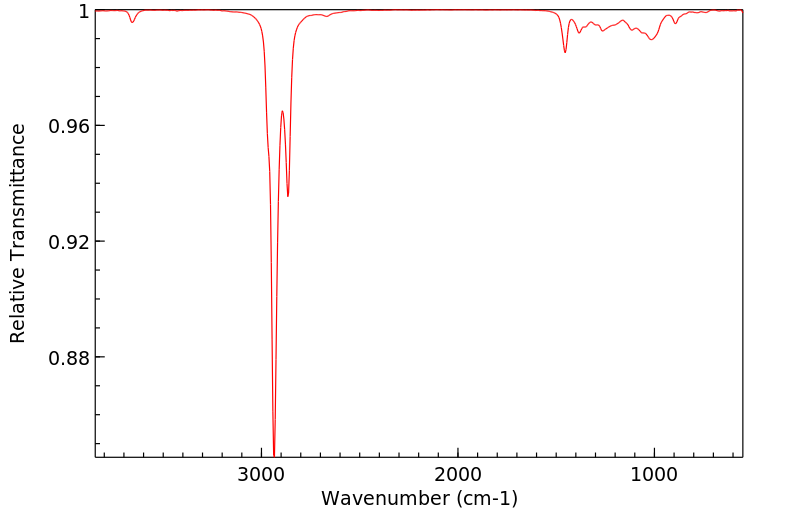
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷