1,2-二(氯甲基)-4,5-二甲苯 | 2362-16-5
中文名称
1,2-二(氯甲基)-4,5-二甲苯
中文别名
——
英文名称
1,2-bis(chloromethyl)-4,5-dimethylbenzene
英文别名
4,5-bis(chloromethyl)-o-xylene;1,2-dimethyl-4,5-bis(chloromethyl)benzene;4,5-Bis(chlormethyl)o-xylol
CAS
2362-16-5
化学式
C10H12Cl2
mdl
MFCD00000911
分子量
203.111
InChiKey
UIMFHDVFMPUGMO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:104 °C
-
沸点:285.8±35.0 °C(Predicted)
-
密度:1.145±0.06 g/cm3(Predicted)
-
保留指数:1542;1530
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903999090
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,4,5-四甲苯 1,2,4,5-tetramethylbenzene 95-93-2 C10H14 134.221
反应信息
-
作为反应物:描述:1,2-二(氯甲基)-4,5-二甲苯 在 1-己基-3-甲基四氟甲烷磺酸咪唑鎓 、 三乙烯二胺 、 copper(II) 2-ethylhexanoate 、 bis(acetylacetonate)oxovanadium 、 氧气 作用下, 120.0 ℃ 、101.33 kPa 条件下, 反应 24.0h, 生成 均苯四甲酸参考文献:名称:An inexpensive and efficient synthetic method for the preparation of pyromellitic dianhyride in ionic liquid摘要:In this article, pyromellitic dianhydride could be successfully obtained in 76.7% total yield by an aerobic oxidation of 1,4-bis(chloromethyl)-2,5-dimethylbenzene or 1,5-bis(chloromethyl)-2,4-dimethylbenzene catalyzed by VO(acac)(2)/Cu(2-Eth)(2)/DABCO in [hmim]OTf and a subsequent dehydration of pyromellitic acid upon heating with acetic anhydride. The starting materials including 1,2-bis(chloromethyl)-4,5-dimethylbenzene were prepared by dichloromethylation of their corresponding xylene catalyzed by [C(12)mim]Br in aqueous media.DOI:10.3998/ark.5550190.0011.907
-
作为产物:描述:参考文献:名称:摘要:By an example of previously uncharacterized products obtained by alkylarenes radical chlorination was demonstrated that combination of various interpretation methods applied to the retention indices (RI) in the gas chromatography on the standard nonpolar phases (comparison of RI of products and initial compounds, characteristics of succession of the chromatographic elution of the structural isomers with the use of estimation of molecular dynamic parameters, application of the additive schemes to RI calculation, and using of structural analogy CH3<----> Cl for testing the results obtained) permitted unambiguous identification of the structure even without data of mass spectrometry.DOI:10.1023/a:1012343416008
文献信息
-
[EN] BENZIMIDAZOLE OR INDOLE AMIDES AS INHIBITORS OF PIN1<br/>[FR] AMIDES DE BENZIMIDAZOLE OU D'INDOLE EN TANT QU'INHIBITEURS DE PIN1申请人:PFIZER公开号:WO2006040646A1公开(公告)日:2006-04-20The invention relates to compounds of the formula (1) and to pharmaceutically acceptable salts and solvates thereof, wherein the variables are defined herein. The invention also relates to methods of treating abnormal cell growth in mammals by administering the compounds of formula (1) and to pharmaceutical compositions for treating such disorders that contain the compounds of formula (1). The invention also relates to methods of preparing the compounds of formula (1).这项发明涉及公式(1)的化合物,以及其药学上可接受的盐和溶剂化合物,其中变量在此处定义。该发明还涉及通过给予公式(1)的化合物来治疗哺乳动物中的异常细胞生长的方法,以及用于治疗包含公式(1)化合物的这类疾病的药物组合物。该发明还涉及制备公式(1)化合物的方法。
-
[EN] SPIRO-OXAZOLONES<br/>[FR] SPIRO-OXAZOLONES申请人:HOFFMANN LA ROCHE公开号:WO2015091411A1公开(公告)日:2015-06-25The present invention provides spiro-oxazolones, which act as V1a receptor modulators, and in particular as V1a receptor antagonists, their manufacture, pharmaceutical compositions containing them and their use as medicaments. The present compounds are useful as therapeutics acting peripherally and centrally in the conditions of inappropriate secretion of vasopressin, anxiety, depressive disorders, obsessive compulsive disorder, autistic spectrum disorders, schizophrenia, aggressive behavior and phase shift sleep disorders, in particular jetlag.
-
Direct Synthesis of Substituted Naphthalenes from 1,3-Dicarbonyl Compounds and 1,2-Bis(halomethyl)benzenes Including a Novel Rearrangement Aromatization of Benzo[<i>c</i>]oxepine作者:Jun-gang Wang、Meng Wang、Jia-chen Xiang、Yan-ping Zhu、Wei-jian Xue、An-xin WuDOI:10.1021/ol302950w日期:2012.12.7unexpected rearrangement aromatization of benzo[c]oxepine has been revealed to synthesize substituted naphthalenes. This observation was further exploited to develop an efficient approach for the construction of naphthalenes from simple and commercially available 1,3-dicarbonyl compounds and 1,2-bis(halomethyl)benzene compounds via a new domino reaction sequence.
-
Methylene-Bridged Glycoluril Dimers: Synthetic Methods作者:Anxin Wu、Arindam Chakraborty、Dariusz Witt、Jason Lagona、Fehmi Damkaci、Marie A. Ofori、Jessica K. Chiles、James C. Fettinger、Lyle IsaacsDOI:10.1021/jo0258958日期:2002.8.1Cyclic ethers 5a,d-f and 25-26 undergo highly diastereoselective dimerization reactions to yield methylene-bridged glycoluril dimers with the formal extrusion of formaldehyde. Last, it is possible to perform selective heterodimerization reactions using both cyclic ethers and glycoluril derivatives bearing ureidyl NH groups. These reactions deliver the desired C- and S-shaped heterodimers with low to moderate亚甲基桥甘脲二聚体是葫芦素(CB [6]),其同系物(CB [n])及其衍生物的基本组成部分。本文介绍了三种互补的合成C型和S型亚甲基桥甘脲二聚体的方法(29-34和37-44)。为此,我们制备了在其凸面上具有多种功能的甘脲衍生物(1a-d)。这些甘脲衍生物在碱性条件下(DMSO,t-BuOK)用1,2-双(卤甲基)芳烃6-15烷基化,得到4a-d和16-24,其中含有一个芳族邻二甲苯基环并可能具有亲核性脲基NH基团。带有潜在亲电环醚基团(5a-f)和25-28的甘脲衍生物是通过各种方法制备的,包括在回流的含多聚甲醛的TFA中进行缩合反应。在大多数情况下,在无水酸性条件下(PTSA,ClCH(2)CH(2)Cl,回流),4a-d和16-24与低聚甲醛的缩合反应可得到C形和S形亚甲基桥接的甘脲高产到高产。在许多情况下,优选以高非对映选择性形成C形化合物。环醚5a,df和25-26经历高度非对
-
Synthesis and Optical Resolution of the Floral Odorant (±)-2,3-Dihydro-2,5-dimethyl-1H-indene-2-methanol, and Preparation of Analogues作者:Christian Vial、Gérald Bernardinelli、Philippe Schneider、Michael Aizenberg、Béat WinterDOI:10.1002/hlca.200590250日期:2005.12(±)-1, a recently discovered, valuable, floral-type odorant, has been synthesized by a straightforward procedure (Scheme 1). To determine the properties of the enantiomers of 1, their separation by preparative HPLC and the determination of their absolute configuration by X-ray crystallography were carried out (Figure). Furthermore, the analogues 2–6 were synthesized, either from differently methylated标题化合物(±)-1是最近发现的,有价值的花香型增香剂,已通过简单的方法合成(方案1)。为了确定1的对映异构体的性质,进行了制备型HPLC分离和X射线晶体学测定其绝对构型(图)。此外,类似物2 - 6合成,或者从不同的甲基化的2-甲基茚-1-酮(方案2和3),或在2,4,6-三甲基化的同系物的情况下6,通过一个完全不同的合成方法(方案4)。对(+)-(S)-1,(-)-(R)-1和(±)-1的评估显示,气味方面仅有微小差异(表)。
表征谱图
-
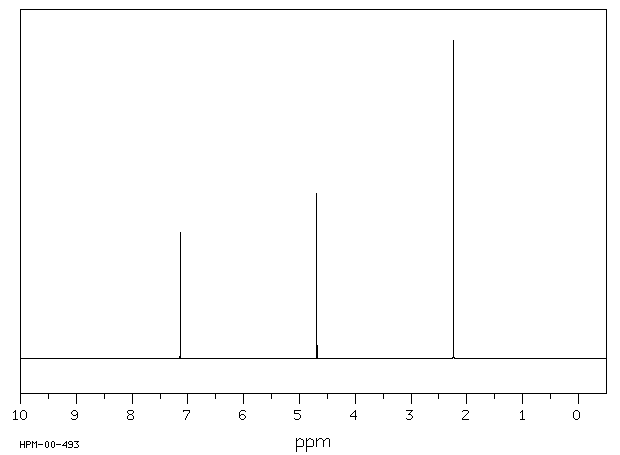
氢谱1HNMR
-
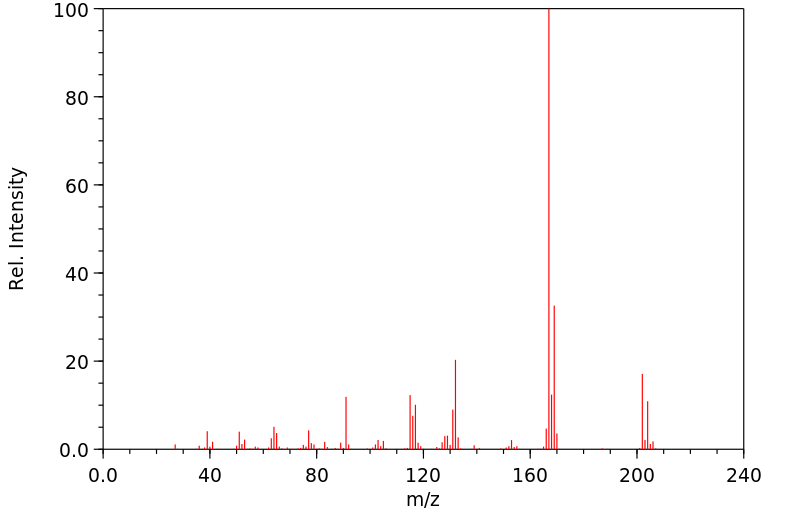
质谱MS
-
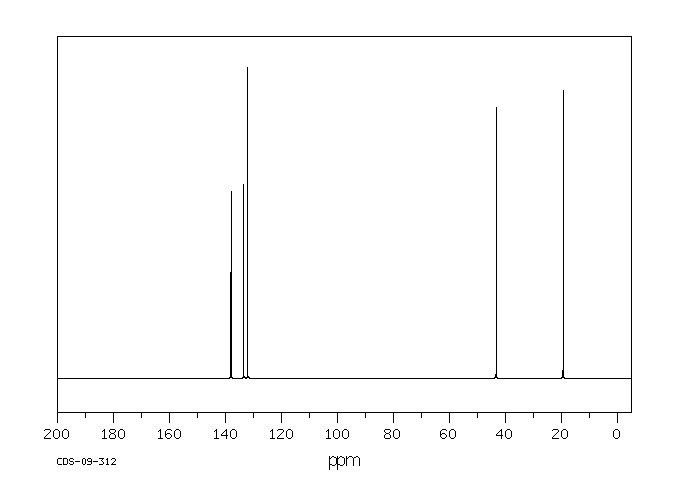
碳谱13CNMR
-
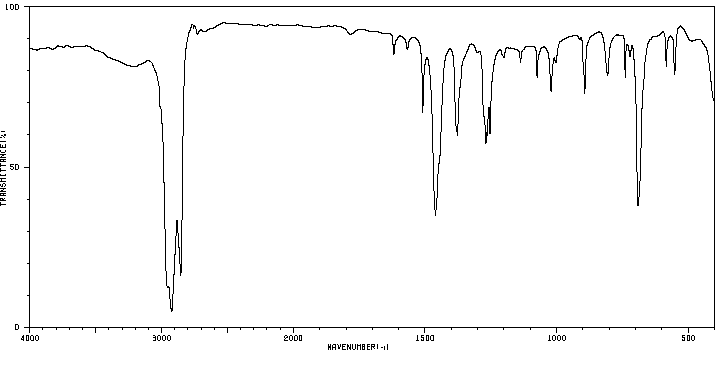
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫