2-溴苄氯 | 578-51-8
中文名称
2-溴苄氯
中文别名
2-溴苯甲基氯;2-溴苄基氯
英文名称
2-bromobenzylchloride
英文别名
1-Bromo-2-(chloromethyl)benzene;o-bromobenzyl chloride;o-Brombenzylchlorid
CAS
578-51-8
化学式
C7H6BrCl
mdl
——
分子量
205.482
InChiKey
DDVSFIUKWUTKES-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:110-111 °C (15 mmHg)
-
密度:1.7341 g/cm3
-
闪点:110-111°C/15mm
-
保留指数:1244;1244
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R34
-
危险品运输编号:3265
-
包装等级:III
-
危险类别:8
-
储存条件:贮存: 将密封的小容器存放在密封的大容器中,并放置在阴凉、干燥的地方。
SDS
| Name: | 2-Bromobenzyl chloride 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 578-51-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 578-51-8 | 2-Bromobenzyl chloride | 98% | unlisted |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.
Potential Health Effects
Eye:
Causes eye burns. Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin burns.
Ingestion:
Causes gastrointestinal tract burns.
Inhalation:
Causes chemical burns to the respiratory tract.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 578-51-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 110 - 111 deg C @15mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H6BrCl
Molecular Weight: 205.48
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 578-51-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Bromobenzyl chloride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S.*
Hazard Class: 8
UN Number: 3265
Packing Group: III
IMO
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3265
Packing Group: III
RID/ADR
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3265
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 578-51-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 578-51-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 578-51-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:2-溴苄氯 在 sodium hydride 、 magnesium 、 1,2-二溴乙烷 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 6.67h, 生成 2-(苯氧甲基)苯甲酸参考文献:名称:Amide-modified prenylcysteine based Icmt inhibitors: Structure–activity relationships, kinetic analysis and cellular characterization摘要:Human protein isoprenylcysteine carboxyl methyltransferase (hIcmt) is the enzyme responsible for the alpha-carboxyl methylation of the C-terminal isoprenylated cysteine of CaaX proteins, including Ras proteins. This specific posttranslational methylation event has been shown to be important for cellular transformation by oncogenic Ras isoforms. This finding led to interest in hIcmt inhibitors as potential anti-cancer agents. Previous analog studies based on N-acetyl-S-farnesylcysteine identified two prenylcysteine-based low micromolar inhibitors (1a and 1b) of hIcmt, each bearing a phenoxyphenyl amide modification. In this study, a focused library of analogs of 1a and 1b was synthesized and screened versus hIcmt, delineating structural features important for inhibition. Kinetic characterization of the most potent analogs 1a and 1b established that both inhibitors exhibited mixed-mode inhibition and that the competitive component predominated. Using the Cheng-Prusoff method, the K-i values were determined from the IC50 values. Analog 1a has a K-IC of 1.4 +/- 0.2 mu M and a K-IU of 4.8 +/- 0.5 mu M while 1b has a K-IC of 0.5 +/- 0.07 mu M and a K-IU of 1.9 +/- 0.2 mu M. Cellular evaluation of 1b revealed that it alters the subcellular localization of GFP-KRas, and also inhibits both Ras activation and Erk phosphorylation in Jurkat cells. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2011.10.087
-
作为产物:参考文献:名称:A Study of the Peroxide-Catalyzed Chlorination of the Bromotoluenes with Sulfuryl Chloride摘要:DOI:10.1021/ja01150a509
文献信息
-
Structure-Based Design, Synthesis, and Biological Evaluation of New Triazolo[1,5-<i>a</i>]Pyrimidine Derivatives as Highly Potent and Orally Active ABCB1 Modulators作者:Shuai Wang、Sai-Qi Wang、Qiu-Xu Teng、Linlin Yang、Zi-Ning Lei、Xiao-Han Yuan、Jun-Feng Huo、Xiao-Bing Chen、Mengru Wang、Bin Yu、Zhe-Sheng Chen、Hong-Min LiuDOI:10.1021/acs.jmedchem.0c01741日期:2020.12.24ABCB1 is a promising therapeutic target for overcoming multidrug resistance (MDR). In this work, we reported the structure-based design of triazolo[1,5-a]pyrimidines as new ABCB1 modulators, of which WS-691 significantly increased sensitization of ABCB1-overexpressed SW620/Ad300 cells to paclitaxel (PTX) (IC50 = 22.02 nM). Mechanistic studies indicated that WS-691 significantly increased the intracellularABCB1是克服多药耐药性(MDR)的有希望的治疗靶标。在这项工作中,我们报道了作为新的ABCB1调节剂的三唑并[1,5- a ]嘧啶的结构设计,其中WS-691显着提高了ABCB1过表达的SW620 / Ad300细胞对紫杉醇(PTX)的敏感性(IC 50 = 22.02 nM)。机理研究表明,WS-691通过抑制ABCB1的外排功能,显着增加PTX和[ 3 H] -PTX的细胞内浓度,同时降低SW620 / Ad300细胞中[ 3 H] -PTX的外排。细胞热位移分析表明,WS-691可通过直接结合ABCB1来稳定ABCB1。WS-691可以刺激ABCB1 ATPase的活性,但对CYP3A4几乎没有抑制活性。重要的是,WS-691在体内没有观察到毒性的情况下提高了SW620 / Ad300细胞对PTX的敏感性。总体而言,WS-691是一种高效且口服的ABCB1调节剂,能够克服MDR。三唑并[1,5-
-
Preparation and Application of Solid, Salt-Stabilized Zinc Amide Enolates with Enhanced Air and Moisture Stability作者:Yi-Hung Chen、Mario Ellwart、Georgios Toupalas、Yusuke Ebe、Paul KnochelDOI:10.1002/anie.201700216日期:2017.4.10various N‐morpholino amides with TMPZnCl⋅LiCl (TMP=2,2,6,6‐tetramethylpiperidyl) and Mg(OPiv)2 in THF at 25 °C provides solid zinc enolates with enhanced air and moisture stability (t1/2 in air: 1–3 h) after solvent evaporation. These enolates undergo Pd‐ and Cu‐catalyzed cross‐couplings with (hetero)aryl bromides as well as allylic and benzylic halides. The arylated N‐morpholino amides were converted into
-
Catalytic Diastereo- and Enantioselective Annulations between Transient Nitrosoalkenes and Indoles作者:Yu Zhang、David Stephens、Graciela Hernandez、Rosalinda Mendoza、Oleg V. LarionovDOI:10.1002/chem.201203435日期:2012.12.21Caught in transition: An efficient catalytic system is the key to the successful development of the first highly diastereo‐ and enantioselective annulation reaction between indoles and transient nitrosoalkenes. This robust reaction affords structurally unique architectures with up to three new chiral centers. The products can be readily elaborated into other indoline‐based chiral heterocyclic motifs
-
Serendipitous base catalysed condensation–heteroannulation of iminoesters: a regioselective route to the synthesis of 4,6-disubstituted 5-azaindoles作者:Premansh Dudhe、Krishnan Venkatasubbaiah、Biswarup Pathak、Venkatesh ChelvamDOI:10.1039/c9ob02657f日期:——A serendipitous discovery of a novel one-pot synthesis of 4,6-disubstituted 5-azaindoles is reported herein. In the presence of Hunig's base, various N-substituted pyrrole-2-carboxaldehydes have been efficiently transformed into their corresponding 4,6-disubstituted 5-azaindoles through an imine mediated cascade condensation-heteroannulation. The synthetic value of the methodology is established by
-
N‐Heterocyclic Carbene Catalyzed Ester Synthesis from Organic Halides through Incorporation of Oxygen Atoms from Air作者:Hui Tan、Shen‐An Wang、Zixi Yan、Jianzhong Liu、Jialiang Wei、Song Song、Ning JiaoDOI:10.1002/anie.202011039日期:2021.1.25Oxygenation reactions with molecular oxygen (O2) as the oxygen source provides a green and straightforward strategy for the construction of O‐containing compounds. Demonstrated here is a novel N‐heterocyclic carbene (NHC) catalyzed oxidative transformation of simple and readily available organic halides into valuable esters through the incorporation of O‐atoms from O2. Mechanistic studies prove that以分子氧(O 2)为氧源的加氧反应为构建含O化合物提供了绿色而直接的策略。这里展示的是一种新颖的N杂环卡宾(NHC)通过将O 2中的O原子并入,将简单易用的有机卤化物催化氧化转化为有价值的酯的方法。机理研究证明,原位产生的脱氧Breslow中间体被氧化为Breslow中间体,通过该氧化方案进一步转化。该方法拓宽了NHC催化领域,并促进了与O 2的氧化反应。
表征谱图
-
氢谱1HNMR
-
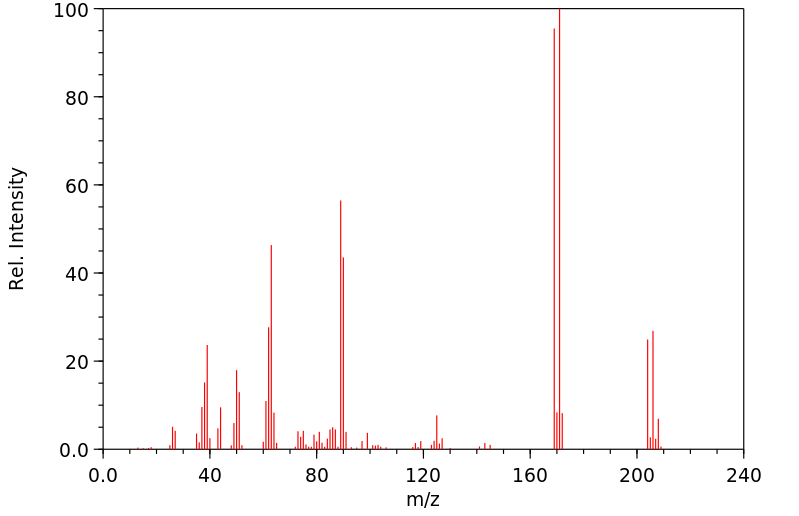
质谱MS
-
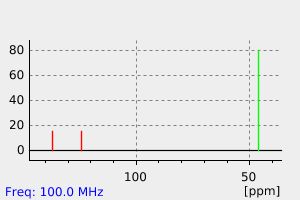
碳谱13CNMR
-
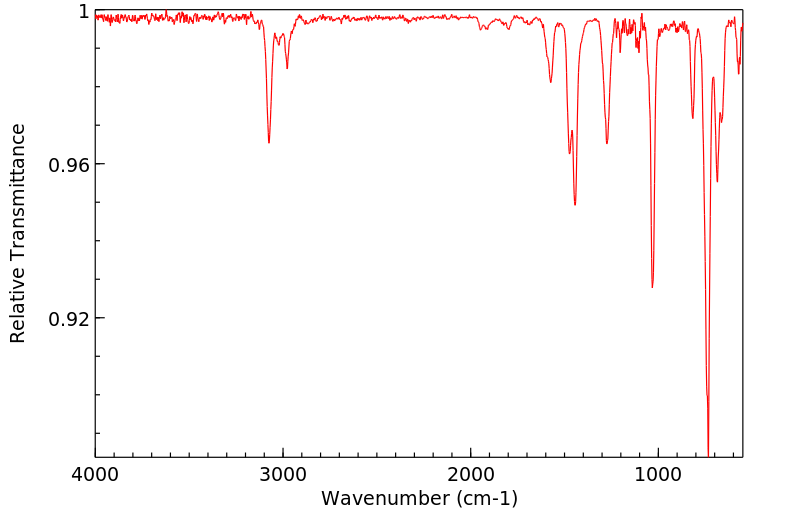
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫