3,4,5-三乙氧基苯甲酸 | 6970-19-0
中文名称
3,4,5-三乙氧基苯甲酸
中文别名
——
英文名称
3,4,5-triethoxybenzoic acid
英文别名
——
CAS
6970-19-0
化学式
C13H18O5
mdl
MFCD00002505
分子量
254.283
InChiKey
YCENSLDYFDZUMO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:109-112 °C
-
沸点:357.49°C (rough estimate)
-
密度:1.2092 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:18
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.461
-
拓扑面积:65
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
安全说明:S24/25,S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2918990090
-
危险性防范说明:P280,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H312,H315,H317,H319,H332
-
储存条件:室温
SDS
| Name: | 3 4 5-Triethoxybenzoic Acid 97+% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 6970-19-0 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6970-19-0 | 3,4,5-Triethoxybenzoic Acid | 97+ | 230-192-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6970-19-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 109-111 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C13H18O5
Molecular Weight: 254.28
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6970-19-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,4,5-Triethoxybenzoic Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 6970-19-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6970-19-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6970-19-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 3,4,5-triethoxybenzoate 39666-77-8 C14H20O5 268.31 没食子酸 3,4,5-trihydroxybenzoic acid 149-91-7 C7H6O5 170.122 没食子酸甲酯 methyl galloate 99-24-1 C8H8O5 184.149 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,5-diethyl-4-methyl gallic acid 61470-52-8 C12H16O5 240.256 —— 3,5-diethoxy-4-hydroxybenzoic acid 91971-67-4 C11H14O5 226.229 —— methyl 3,4,5-triethoxybenzoate 39666-77-8 C14H20O5 268.31 乙基3,4,5-三乙氧基苯甲酸酯 3,4,5-triethoxybenzoic acid ethyl ester 31374-71-7 C15H22O5 282.337 —— 3,4,5-triethoxybenzaldehyde 860724-22-7 C13H18O4 238.284 3,4,5-三乙氧基苄醇 3,4,5-triethoxybenzyl alcohol 39727-75-8 C13H20O4 240.299 —— 3.4.5-Triaethoxy-benzoesaeure-<2-(2-chlor-aethylamino)-aethylester> —— C17H26ClNO5 359.85 —— 3,4,5-triethoxy-benzoic acid-(2-diethylamino-ethyl ester) 102708-87-2 C19H31NO5 353.459 —— 3,4,5-triethoxy-benzoic acid amide 349115-20-4 C13H19NO4 253.298 —— 3,4,5-Triethoxyacetophenon 1147-81-5 C14H20O4 252.31 —— 3.4.5-Triaethoxy-toluol 39727-76-9 C13H20O3 224.3 —— 3,4,5-triethoxybenzoyl chloride 50915-97-4 C13H17ClO4 272.729 3,4,5-三乙氧基苯并肼 3,4,5-triethoxybenzoic acid hydrazide 379254-36-1 C13H20N2O4 268.313 —— 3',4',5'-triethoxypropiophenone 184963-78-8 C15H22O4 266.337 —— 3,4,5-Triethoxybenzoyl isothiocyanate 1154010-69-1 C14H17NO4S 295.359 —— 3,4,5-triethoxy-benzonitrile —— C13H17NO3 235.283 —— 3,4,5-triethoxy-benzoyl azide 49541-84-6 C13H17N3O4 279.296 —— 2-bromo-3,4,5-triethoxybenzoic acid 911701-67-2 C13H17BrO5 333.179 —— 3,4,5-triethoxyphenethylamine 90109-63-0 C14H23NO3 253.342 —— N.N-Bis-<2-chlor-aethyl>-3.4.5-triaethoxy-benzylamin —— C17H27Cl2NO3 364.312 —— 5-(4-methylphenyl)-2-furylmethyl 3,4,5-triethoxybenzoate 1365714-28-8 C25H28O6 424.494 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Ouinone derivatives and pharmacological use摘要:一种用于治疗肝脏疾病的醌衍生物,其通用公式为:##STR1## 其中R1选自由烷基、烯基、炔基或杂环组成的组,R2是含有取代氮原子的基团,所述氮原子上的取代基选自由氢、取代或不取代的低级烷基或杂环组成的组,并且所述氮原子可以是环杂原子,而R3、R4和R5可以是相同的或不同的,且每个都是氢、低级烷基或低级烷氧基团。公开号:US05210239A1
-
作为产物:描述:没食子酸甲酯 在 potassium carbonate 、 potassium hydroxide 作用下, 以 水 、 N,N-二甲基甲酰胺 、 异丙醇 为溶剂, 生成 3,4,5-三乙氧基苯甲酸参考文献:名称:扩展 2-苯基苯并碲唑库:乙氧基官能团对紫外/可见光吸收特性的红移效应摘要:这项工作描述了在 2 位带有苯环的新型苯并碲唑系列的高产率合成,该苯环被不同的乙氧基链官能化。改变这些官能团的数量和位置决定了固态自组装的差异,以及目标分子的光物理特性。正如对每个分子的 HOMO-LUMO 间隙的理论计算所预期的那样,与未取代的苯并碲唑相比,邻位和对位乙氧基链的存在决定了吸收峰中高达 20 nm 的红移。同样,对于那些带有o的衍生物,在125 Te NMR 光谱的化学位移中观察到更显着的变化。-和对-乙氧基官能化。DOI:10.1055/s-0041-1737898
文献信息
-
A Rhodium-Catalyzed Cascade Cyclization: Direct Synthesis of<i>N</i>-Substituted Phthalimides from Isocyanates and Benzoic Acids作者:Xian-Ying Shi、Andrea Renzetti、Soumen Kundu、Chao-Jun LiDOI:10.1002/adsc.201300834日期:2014.3.10A rhodium(III)‐catalyzed amidation between benzoic acids and isocyanates via direct functionalization of an ortho CH bond followed by intramolecular cyclization is described. This cascade cyclization affords N‐substituted phthalimides in one step in 26–91% yields. The reaction is highly atom‐economical, since no theoretical waste except for water is generated in the reaction.
-
Rh<sup>III</sup>-Catalyzed C−H Olefination of Benzoic Acids under Mild Conditions using Oxygen as the Sole Oxidant作者:Quandi Jiang、Changlei Zhu、Huaiqing Zhao、Weiping SuDOI:10.1002/asia.201500601日期:2016.2Phthalide skeletons have been synthesized for the first time through a RhIII‐catalyzed C−H olefination of benzoic acids under mild conditions using oxygen as the sole oxidant. Aromatic acids bearing a variety of functional groups could react with diverse alkenes to afford the desired cyclized lactones or uncyclized alkenylarenes in moderate‐to‐excellent yields.
-
Depigmenting Activity and Low Cytotoxicity of Alkoxy Benzoates or Alkoxy Cinnamte in Cultured Melanocytes作者:Hak Hee Kang、Ho Sik Rho、Jae Sung Hwang、Seong-Geon OhDOI:10.1248/cpb.51.1085日期:——To obtain effective and safe topical depigmenting agents, we synthesized hydroxybenzoates, alkoxybenzoates, and 3,4,5-trimethoxycinnamate containing a thymol moiety and screened then for high-level inhibitory activity against melanin synthesis in cultured melanocytes. Eight compounds were tested for their depigmenting effect and cytotoxicity using a murine melanocyte cell line. We found that 3,4,5-trialkoxybenzoates and 3,4,5-trimethoxycinnamate, synthesized by conjugating 3,4,5-trialkoxybenzoic acids and 3,4,5-trimethoxycinnmic acid with thymol, showed a potent depigmenting effect and low cytotoxicity. Compound 4h, 5-methyl-2-(methylethyl)phenyl (2E)-3-(3,4,5-trimethoxyphenyl)prop-2-enoate, showed the most potent depigmenting effect (IC50=10 μM) with low cytotoxicity (IC50=200 μM).为了获得有效且安全的局部脱色剂,我们合成了含百里酚基团的羟基苯甲酸盐、烷氧基苯甲酸盐和3,4,5-三甲氧基肉桂酸盐,并对其在培养的黑素细胞中抑制黑色素合成的高水平活性进行了筛选。我们使用小鼠黑素细胞系测试了八种化合物的脱色效果和细胞毒性。研究发现,通过将3,4,5-三烷氧基苯甲酸和3,4,5-三甲氧基肉桂酸与百里酚结合合成的3,4,5-三烷氧基苯甲酸盐和3,4,5-三甲氧基肉桂酸盐显示出强大的脱色效果和低细胞毒性。化合物4h,5-甲基-2-(甲基乙基)苯基(2E)-3-(3,4,5-三甲氧基苯基)丙-2-烯酸酯,显示出最强的脱色效果(IC50=10 μM)和低细胞毒性(IC50=200 μM)。
-
ANTINEOPLASTIC AGENTS: IX. N-BENZYL-N-BIS(2-HALOETHYL)AMINES作者:George R. Pettit、David S. Blonda、Ernest C. HarringtonDOI:10.1139/v63-435日期:1963.12.1and three N-bis(2-iodoethyl)benzylamines have been prepared for cancer chemotherapy studies. The chloro-nitrogen mustards were readily obtained by aluminum chloride – lithium aluminum hydride reduction of the corresponding N-bis(2-chloroethyl)amides. A convenient procedure for converting N-bis(2-chloroethyl)-benzylamine hydrochlorides to N-bis(2-iodoethyl)benzylamine hydroiodides, employing sodium
-
Design of Trehalose‐Based Amide/Sulfonamide C‐type Lectin Receptor Signaling Compounds作者:Omer K. Rasheed、Cassandra Buhl、Jay T. Evans、Kendal T. RyterDOI:10.1002/cmdc.202000775日期:2021.4.20Mycobacterium tuberculosis. As an expansion of our previous work, a library of 6,6′‐amide and sulfonamide α,α‐d‐trehalose compounds with various substituents on the aromatic ring was synthesized efficiently in good to excellent yields. These compounds were evaluated for their ability to activate the human C‐type lectin receptor Mincle by the induction of cytokines from human peripheral blood mononuclear cellsMincle 激动剂已被证明可以诱导炎症细胞因子的产生,例如肿瘤坏死因子-α (TNF),并促进 Th1/Th17 免疫反应的发展,这对于开发针对结核分枝杆菌等病原体的有效疫苗接种可能至关重要。作为我们之前工作的扩展,我们有效地合成了芳环上具有各种取代基的 6,6'-酰胺和磺酰胺 α,α- d -海藻糖化合物库,收率良好至优异。评估了这些化合物通过诱导人外周血单核细胞产生的细胞因子来激活人 C 型凝集素受体 Mincle 的能力。这些新型海藻糖二酰胺和磺酰胺的初步构效关系 (SAR) 表明,与 Mincle 配体海藻糖二山嵛酸酯佐剂 (TDB) 和天然配体海藻糖二霉菌酸酯相比,芳基酰胺连接的海藻糖化合物表现出改善的活性和相对高效的细胞因子产生(TDM) 在 HEK 报告细胞系中诱导剂量依赖性和人类 Mincle 特异性刺激。
表征谱图
-
氢谱1HNMR
-
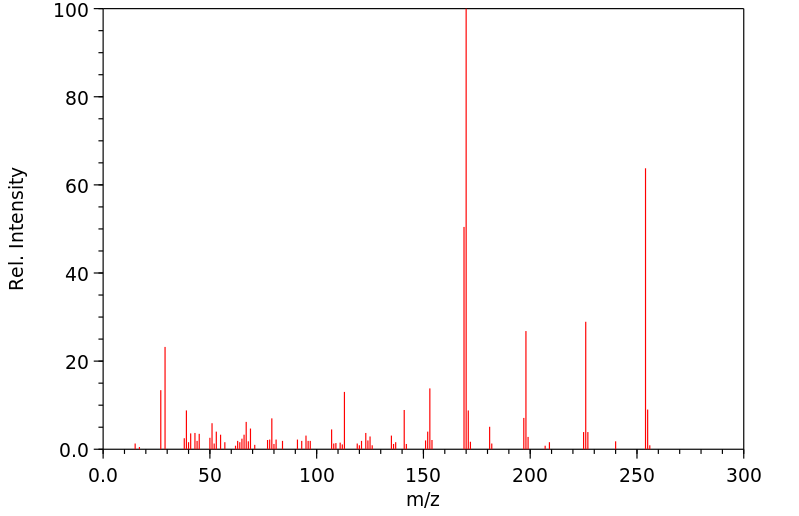
质谱MS
-
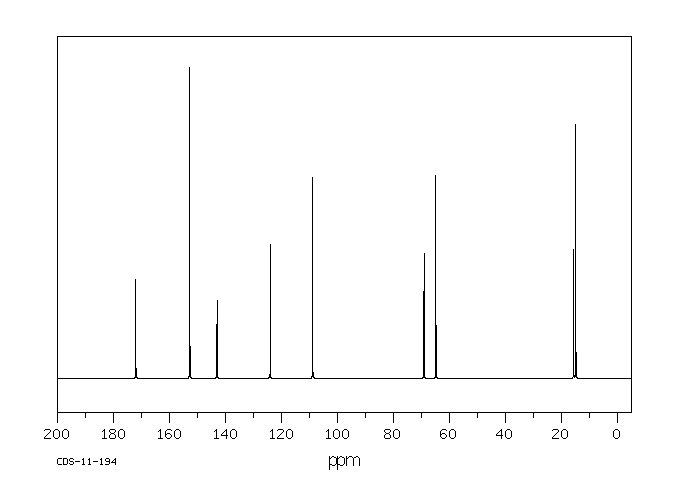
碳谱13CNMR
-
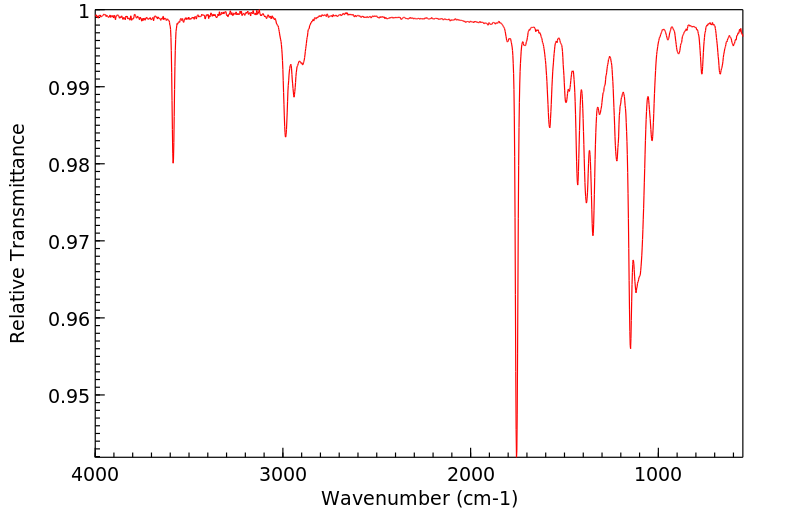
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫