3-乙基-2-甲基-1-戊烯 | 19780-66-6
中文名称
3-乙基-2-甲基-1-戊烯
中文别名
——
英文名称
3-ethyl-2-methyl-1-pentene
英文别名
3-ethyl-2-methyl-pent-1-ene;3-Aethyl-2-methyl-pent-1-en;2-Methyl-3-aethyl-penten (1);2-Methyl-3-aethyl-penten-(1);2-Methyl-3-ethyl-1-penten;2-Methyl-3-aethylpenten;1-Pentene, 3-ethyl-2-methyl-;3-ethyl-2-methylpent-1-ene
CAS
19780-66-6
化学式
C8H16
mdl
MFCD00048664
分子量
112.215
InChiKey
HPHHYSWOBXEIRG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-112.9°C
-
沸点:109.55°C
-
密度:0.72
-
LogP:4.330 (est)
-
保留指数:754.3;735
-
稳定性/保质期:
避免接触强氧化制剂。
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
海关编码:2901299090
-
包装等级:II
-
危险类别:3
-
危险品运输编号:UN 3295
-
储存条件:在密封的贮藏器中存放,并将其置于阴凉、干燥处。
SDS
反应信息
-
作为反应物:描述:3-乙基-2-甲基-1-戊烯 在 dihydrogen hexachloroplatinate 、 三氯硅烷 作用下, 以 四氢呋喃 、 苯 为溶剂, 110.0 ℃ 、9.81 MPa 条件下, 生成 trichloro-(3-ethyl-2-methylpentyl)silane参考文献:名称:Pinazzi,Ch.P. et al., Bulletin de la Societe Chimique de France, 1974, p. 2166 - 2170摘要:DOI:
-
作为产物:描述:参考文献:名称:丙二酰亚胺与单取代或双取代烯烃的对映选择性 [2+2] 环加成摘要:以手性N , N'-二氧化镁(II)络合物为催化剂,实现了烯丙基亚胺与烯烃的高效催化不对称[2+2]环加成反应。该方案提供了一系列高收率且具有出色对映选择性的轴向手性环丁烯。基于实验研究和 DFT 计算提出了逐步机制,π-π 堆积相互作用对对映选择性至关重要。DOI:10.1002/anie.202211596
文献信息
-
Site-selective amination towards tertiary aliphatic allylamines作者:Shengchun Wang、Yiming Gao、Zhao Liu、Demin Ren、He Sun、Linbin Niu、Dali Yang、Dongchao Zhang、Xing’an Liang、Renyi Shi、Xiaotian Qi、Aiwen LeiDOI:10.1038/s41929-022-00818-y日期:——amination of olefins with secondary alkyl amines to afford allylic amines, eliminating the need for oxidants. This method is compatible with a broad scope of olefins and can be extended to achieve a site- and diastereoselective amination of terpenes. Mechanistic studies disclose that the reaction proceeds via a cobaloxime-promoted hydrogen atom transfer pathway to afford the product that results from cleavage
-
Iron<sup>III</sup>-catalyzed asymmetric inverse-electron-demand hetero-Diels–Alder reaction of dioxopyrrolidines with simple olefins作者:Tangyu Zhan、Liang Zhou、Yuqiao Zhou、Bingqian Yang、Xiaoming Feng、Xiaohua LiuDOI:10.1039/d4sc00078a日期:——hetero-Diels–Alder reaction of dioxopyrrolidines with a variety of simple olefins has been accomplished, significantly expanding the applicability of this cyclization to both cyclic hetero-dienes and dienophiles. A new type of strong Lewis acid catalyst of ferric salt enables the LUMO activation of dioxopyrrolidines via formation of cationic species, this method yields a range of bicyclic dihydropyran derivatives
-
Pinazzi,C.P. et al., Bulletin de la Societe Chimique de France, 1975, p. 201 - 205作者:Pinazzi,C.P. et al.DOI:——日期:——
-
Temperature dependence of polar and steric effects in CCl2 cycloadditions作者:Berndt Giese、Carola NeumannDOI:10.1016/s0040-4039(00)87668-6日期:1982.1
-
CN117003604申请人:——公开号:——公开(公告)日:——
表征谱图
-
氢谱1HNMR
-
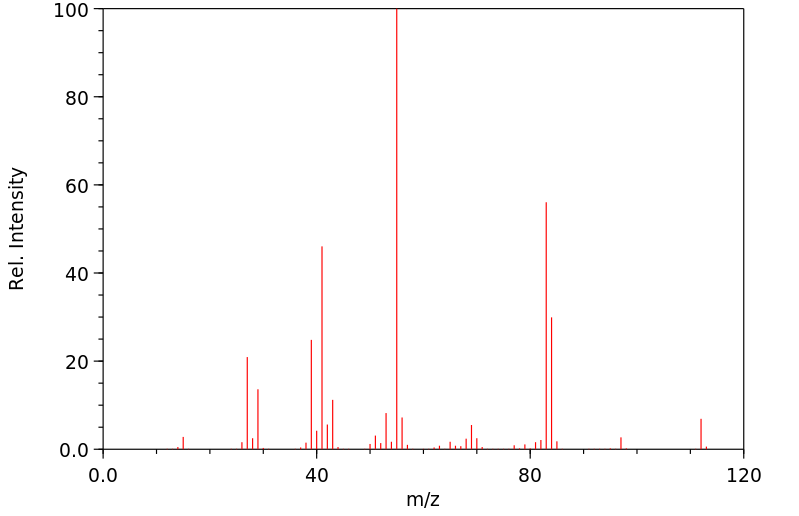
质谱MS
-
碳谱13CNMR
-
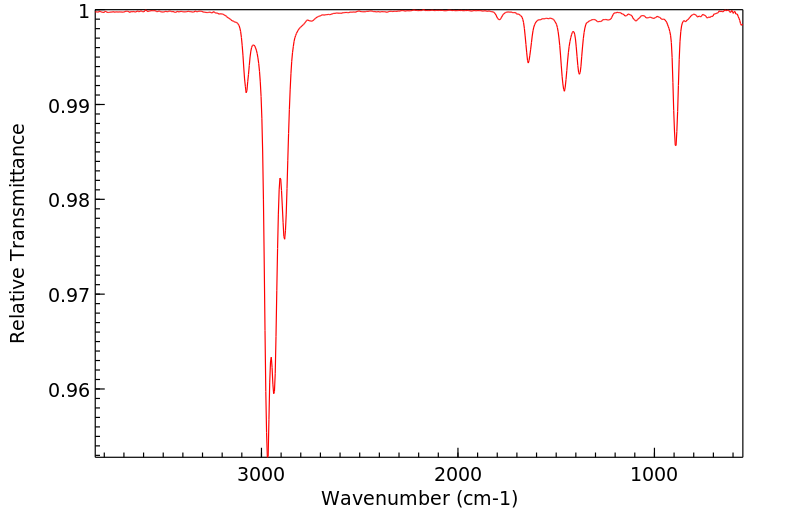
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-