5-苯氧基戊烷基溴 | 22921-72-8
中文名称
5-苯氧基戊烷基溴
中文别名
5-苯氧基戊基溴;5-溴戊基苯醚
英文名称
(5-bromopentoxy)benzene
英文别名
((5-bromopentyl)oxy)benzene;1-bromo-5-phenoxypentane;5-phenoxy-1-bromopentane;5-Phenoxypentyl Bromide;5-bromopentoxybenzene
CAS
22921-72-8
化学式
C11H15BrO
mdl
——
分子量
243.143
InChiKey
WNGRHTGNGQSCTL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:160-165 °C(Press: 11 Torr)
-
密度:1.262±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:13
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.45
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2909309090
-
储存条件:室温
SDS
5-苯氧基戊基溴
模块 1. 化学品
产品名称: 5-Phenoxyamyl Bromide
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5-苯氧基戊基溴
百分比: >95.0%(GC)
CAS编码: 22921-72-8
俗名: 5-PhenoxypeNTyl Bromide , 5-Bromoamyl Phenyl Ether , 5-BromopeNTyl Phenyl
Ether
分子式: C11H15BrO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
5-苯氧基戊基溴
模块 5. 消防措施
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 130 °C/0.3kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.28
溶解度:
[水] 无资料
5-苯氧基戊基溴
模块 9. 理化特性
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
5-苯氧基戊基溴
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 5-Phenoxyamyl Bromide
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5-苯氧基戊基溴
百分比: >95.0%(GC)
CAS编码: 22921-72-8
俗名: 5-PhenoxypeNTyl Bromide , 5-Bromoamyl Phenyl Ether , 5-BromopeNTyl Phenyl
Ether
分子式: C11H15BrO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
5-苯氧基戊基溴
模块 5. 消防措施
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 130 °C/0.3kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.28
溶解度:
[水] 无资料
5-苯氧基戊基溴
模块 9. 理化特性
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
5-苯氧基戊基溴
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 5-phenoxypentan-1-ol 16654-52-7 C11H16O2 180.247 —— 6-phenoxyhexan-1-ol 16654-54-9 C12H18O2 194.274 —— (pent-4-en-1-yloxy)benzene 2653-86-3 C11H14O 162.232 5-苯氧基正戊酸 5-phenoxyvaleric acid 7170-40-3 C11H14O3 194.23 3-苯氧基溴丙烷 3-bromophenoxypropane 588-63-6 C9H11BrO 215.09 乙基5-苯氧基戊酸酯 ethyl 5-(phenoxy)pentanoate 69687-95-2 C13H18O3 222.284 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 戊氧基苯 (pentyloxy)benzene 2050-04-6 C11H16O 164.247 —— 1-bromo-7-phenoxyheptane 51795-98-3 C13H19BrO 271.197 7-苯氧基庚烷-1-醇 7-phenoxyheptan-1-ol 16654-56-1 C13H20O2 208.301 —— 9-phenoxy-1-nonanol 1192596-26-1 C15H24O2 236.354 —— 6-Phenoxyhexylamine 16728-68-0 C12H19NO 193.289 6-苯氧基己腈 6-phenoxyhexanenitrile 16728-57-7 C12H15NO 189.257 —— 5-Phenoxypentylmethylamine 361395-25-7 C12H19NO 193.289 —— 5-Phenoxy-pentylhydrazin 69782-08-7 C11H18N2O 194.277 —— 1-phenoxy-7-nonanol 126435-91-4 C15H24O2 236.354 —— 6-phenoxyhexanoic acid 7170-41-4 C12H16O3 208.257 —— dimethyl-(5-phenoxy-pentyl)-amine 71933-96-5 C13H21NO 207.316 7-苯氧基-庚酸 7-Phenoxy-heptansaeure 7170-42-5 C13H18O3 222.284 —— 2-methyl-8-phenoxy-1-octene 257290-48-5 C15H22O 218.339 —— N-methyl-N-(5-phenoxypentyl)-ethylamine —— C14H23NO 221.343 —— dimethyl-(7-phenoxy-heptyl)-amine 71933-98-7 C15H25NO 235.37 —— diethyl-(5-phenoxy-pentyl)-amine 32599-74-9 C15H25NO 235.37 —— 1-(5-phenoxy-pentyl)-piperidine 86355-72-8 C16H25NO 247.381 —— 1-(5-phenoxy-pentyl)-pyrrolidine 220727-99-1 C15H23NO 233.354 —— N-(5-phenoxypentyl)-hexamethyleneimine —— C17H27NO 261.407 —— ethyl 7-(phenoxy)heptanoate 857787-75-8 C15H22O3 250.338 —— 1-(5-Phenoxypentyl)piperazine 401485-52-7 C15H24N2O 248.368 —— 3-[4-(5-Phenoxypentyl)piperazin-1-yl]propan-1-amine 1383806-31-2 C18H31N3O 305.464 —— 5-phenoxypentyl methacrylate 1313240-69-5 C15H20O3 248.322 —— (5-phenoxy-pentyl)-malonic acid 92865-48-0 C14H18O5 266.294 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Gaubert; Linstead; Rydon, Journal of the Chemical Society, 1937, p. 1977摘要:DOI:
-
作为产物:参考文献:名称:CYCLIC QUATERNARY AMMONIUM SALTS FROM HALOGENATED ALIPHATIC TERTIARY AMINES摘要:DOI:10.1021/ja01364a041
文献信息
-
[EN] 2-SULFANYL-BENZOIMIDAZOL-1-YL-ACETIC ACID DERIVATIVES AS CRTH2 ANTAGONISTS<br/>[FR] DERIVES DE L'ACIDE 2-SULFANYL-BENZOIMIDAZOL-1-YL-ACETIQUE EN TANT QU'ANTAGONISTES DE CRTH2申请人:ACTELION PHARMACEUTICALS LTD公开号:WO2006021418A1公开(公告)日:2006-03-02The invention relates to 2-sulfanyl-benzoimidazol-1-yl-acetic acid derivatives and their use as potent 'chemoattractant receptor-homologous molecule expressed on Th2 cells' antagonists in the treatment of prostaglandin mediated diseases, to pharmaceutical compositions containing these derivatives and to processes for their preparation.
-
[EN] 2-CYCLOALKYL RESORCINOL CANNABINERGIC LIGANDS<br/>[FR] LIGANDS CANNABINERGIQUES DE 2-CYCLOALKYL RÉSORCINOL申请人:UNIV NORTHEASTERN公开号:WO2014062965A1公开(公告)日:2014-04-24The present invention relates to novel 2-cycloalkyl resorcinol compounds; to pharmaceutical compositions comprising the compounds; and to methods of preparing the compounds and uses thereof. The disclosed compounds can bind to and modulate the cannabinoid receptors and thus, they are specific ligands for these receptors. The invented compounds, when administered in a therapeutically effective amount to an individual or animal, results in a sufficiently high level of that compound in the individual or animal to cause a physiological response. The physiological response may be useful to treat a number of physiological conditions.
-
Silver-Catalyzed Trifluoromethoxylation of Alkyl Trifluoroborates作者:Xiaohuan Jiang、Pingping TangDOI:10.1021/acs.orglett.0c01741日期:2020.7.2A silver-catalyzed trifluoromethoxylation of alkyl trifluoroborates with trifluoromethyl arylsulfonate as the trifluoromethoxylation reagent has been reported for the first time. This reaction is performed under mild reaction conditions and has wide functional group compatibility. In addition, the mechanism of this site-specific trifluoromethoxylation is proposed as a radical pathway.
-
Leukotriene LTD.sub.4 and LTB.sub.4 antagonists申请人:G. D. Searle & Co.公开号:US04808729A1公开(公告)日:1989-02-28The invention encompasses compounds of Formula I ##STR1## and the pharmaceutically acceptable salts and geometrical and optical isomers thereof wherein: X, Y, and Z are each independently O or S with S optionally oxidized to S.dbd.O; Alk is alkylene or hydroxyalkylene containing 1-6 carbon atoms; R.sub.1 is hydrogen or lower alkyl; n is 0 to 5; R.sub.2 is hydrogen, lower alkyl, cycloalkyl, --(CH.sub.2).sub.n --CO.sub.2 R.sub.1, phenyl, phenyl substituted with halo, lower alkyl or lower alkoxy; and Ar is 5,6,7,8-tetrahydro-1-naphthalenyl, phenyl, or phenyl substituted with lower alkyl, hydroxy, lower alkoxy, or lower alkanoyl. The compounds are useful as anti-allergy agents and anti-inflammatory agents.该发明涵盖了式I的化合物及其药用可接受的盐和几何和光学异构体,其中:X、Y和Z分别独立地为O或S,其中S可选择性地氧化为S.dbd.O;Alk为含有1-6个碳原子的烷基或羟基烷基;R.sub.1为氢或较低的烷基;n为0至5;R.sub.2为氢、较低的烷基、环烷基、--(CH.sub.2).sub.n --CO.sub.2 R.sub.1、苯基、用卤素取代的苯基、较低的烷基或较低的烷氧基;Ar为5,6,7,8-四氢-1-萘基、苯基或用较低的烷基、羟基、较低的烷氧基或较低的烷酰基取代的苯基。这些化合物可用作抗过敏剂和抗炎剂。
-
Design of PAP-1, a Selective Small Molecule Kv1.3 Blocker, for the Suppression of Effector Memory T Cells in Autoimmune Diseases作者:Alexander Schmitz、Ananthakrishnan Sankaranarayanan、Philippe Azam、Kristina Schmidt-Lassen、Daniel Homerick、Wolfram Hänsel、Heike WulffDOI:10.1124/mol.105.015669日期:2005.11The lymphocyte K+ channel Kv1.3 constitutes an attractive pharmacological target for the selective suppression of terminally differentiated effector memory T (TEM) cells in T cell-mediated autoimmune diseases, such as multiple sclerosis and type 1 diabetes. Unfortunately, none of the existing small-molecule Kv1.3 blockers is selective, and many of them, such as correolide, 4-phenyl-4-[3-(methoxyphenyl)-3-oxo-2-azapropyl]cyclohexanone, and our own compound Psora-4 inhibit the cardiac K+ channel Kv1.5. By further exploring the structure-activity relationship around Psora-4 through a combination of traditional medicinal chemistry and whole-cell patch-clamp, we identified a series of new phenoxyalkoxypsoralens that exhibit 2- to 50-fold selectivity for Kv1.3 over Kv1.5, depending on their exact substitution pattern. The most potent and “drug-like” compound of this series, 5-(4-phenoxybutoxy)psoralen (PAP-1), blocks Kv1.3 in a use-dependent manner, with a Hill coefficient of 2 and an EC50 of 2 nM, by preferentially binding to the C-type inactivated state of the channel. PAP-1 is 23-fold selective over Kv1.5, 33- to 125-fold selective over other Kv1-family channels, and 500- to 7500-fold selective over Kv2.1, Kv3.1, Kv3.2, Kv4.2, HERG, calcium-activated K+ channels, Na+,Ca2+, and Cl- channels. PAP-1 does not exhibit cytotoxic or phototoxic effects, is negative in the Ames test, and affects cytochrome P450-dependent enzymes only at micromolar concentrations. PAP-1 potently inhibits the proliferation of human TEM cells and suppresses delayed type hypersensitivity, a TEM cell-mediated reaction, in rats. PAP-1 and several of its derivatives therefore constitute excellent new tools to further explore Kv1.3 as a target for immunosuppression and could potentially be developed into orally available immunomodulators.淋巴细胞K+通道Kv1.3是选择性抑制T细胞介导的自身免疫性疾病(如多发性硬化症和1型糖尿病)中终末分化效应记忆T(TEM)细胞的有吸引力的药理学靶点。不幸的是,目前存在的所有小分子Kv1.3阻断剂均不具备选择性,其中许多如柯里奥利德、4-苯基-4-[3-(甲氧苯基)-3-酮-2-氮杂丙基]环己酮以及我们自己的化合物Psora-4均可抑制心脏K+通道Kv1.5。通过结合传统药物化学和全细胞膜片钳技术进一步探索Psora-4的构效关系,我们鉴定了一系列新的苯氧烷氧基补骨脂素,它们对Kv1.3的选择性比Kv1.5高2至50倍,具体取决于其取代模式。这一系列化合物中,最具有强效和“类药物”性质的化合物为5-(4-苯氧基丁氧基)补骨脂素(PAP-1),它以使用依赖性方式阻断Kv1.3,Hill系数为2,EC50为2 nM,通过优先结合通道的C型失活态。PAP-1对Kv1.5的选择性为23倍,对其他Kv1家族通道的选择性为33至125倍,对Kv2.1、Kv3.1、Kv3.2、Kv4.2、HERG、钙激活K+通道、Na+、Ca2+及Cl-通道的选择性为500至7000倍。PAP-1无细胞毒性或光毒性,Ames试验呈阴性,对细胞色素P450依赖性酶的影响仅在微摩尔浓度下显现。PAP-1能强效抑制人TEM细胞的增殖并抑制大鼠中的迟发型超敏反应(一种TEM细胞介导的反应)。因此,PAP-1及其若干衍生物构成了探索Kv1.3作为免疫抑制靶点的新工具,并有可能开发成口服可用的免疫调节剂。
表征谱图
-
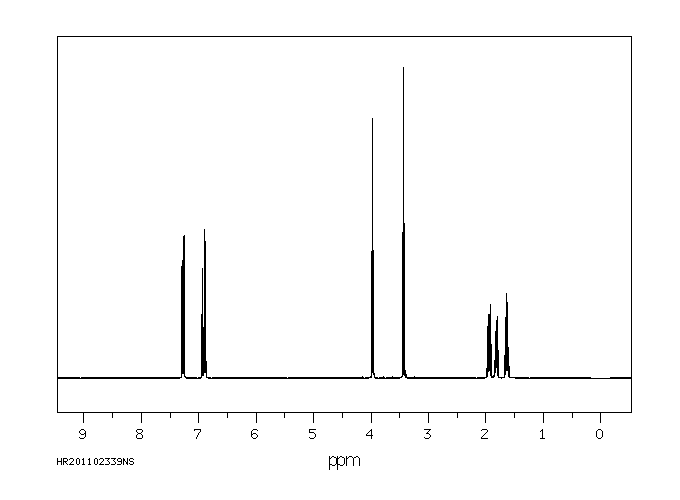
氢谱1HNMR
-
质谱MS
-
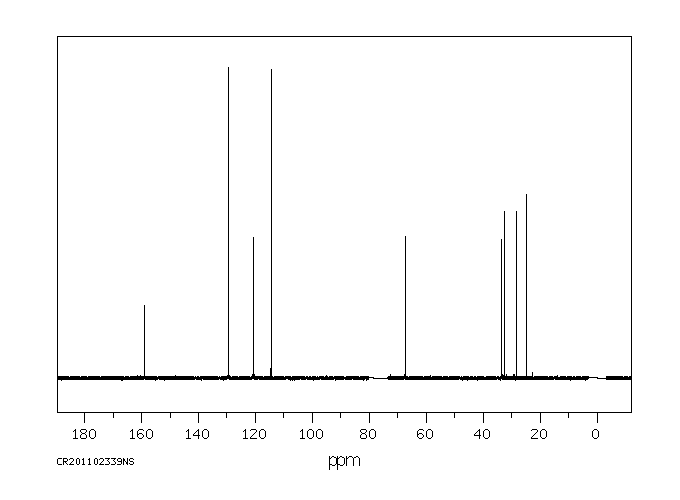
碳谱13CNMR
-
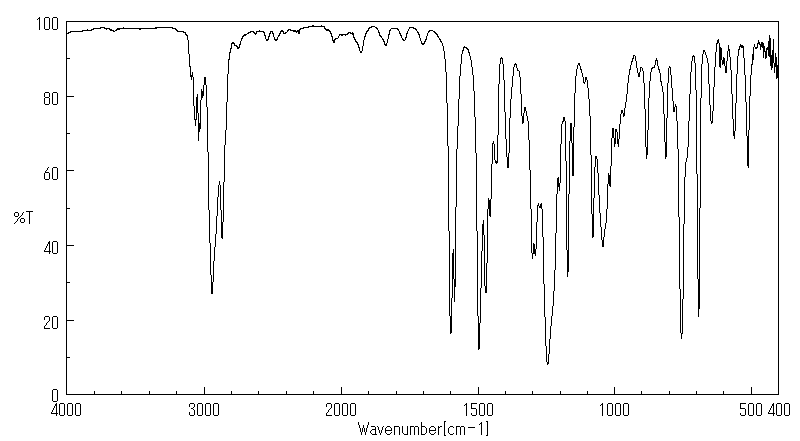
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯