月桂醇月桂酸酯 | 13945-76-1
中文名称
月桂醇月桂酸酯
中文别名
月桂酸月桂醇酯
英文名称
dodecyl laurate
英文别名
dodecyl dodecanoate;lauryl laurate;dodecanoic acid dodecyl ester
CAS
13945-76-1
化学式
C24H48O2
mdl
——
分子量
368.644
InChiKey
CYUUZGXOQDCCGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:27 °C
-
沸点:226 °C
-
密度:0.860±0.06 g/cm3(Predicted)
-
LogP:10.975 (est)
-
保留指数:2554.05;2553
计算性质
-
辛醇/水分配系数(LogP):10.9
-
重原子数:26
-
可旋转键数:22
-
环数:0.0
-
sp3杂化的碳原子比例:0.96
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
WGK Germany:3
-
海关编码:2915900090
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H312,H315,H319,H332,H335
-
储存条件:室温
SDS
制备方法与用途
用途
月桂醇月桂酸酯可广泛应用于化妆品等领域。
上下游信息
反应信息
-
作为反应物:参考文献:名称:Pt-MoOx / TiO2催化剂将脂肪酸,甘油三酸酯和酮加氢脱氧为液态烷烃摘要:筛选了各种负载型金属催化剂,用于月桂酸和2-辛酮的加氢反应,以作为模型反应,在无溶剂条件下在间歇式反应器中将生物质衍生的含氧化合物转化为液态烷烃(生物燃料)。在所测试的催化剂中,共载于TiO 2上的Pt和MoO x(Pt–MoO x / TiO 2)对于两种反应均显示出最高的正烷烃收率。在50 bar H 2下,Pt–MoO x / TiO 2选择性催化各种脂肪酸和甘油三酸酯加氢脱氧为正烷烃,而没有C–C键断裂并且显示出比文献中的催化剂更高的周转率。Pt–MoO x / TiO 2对各种酮加氢脱氧为相应的烷烃也有效。H 2下吸附丙酮反应的原位红外研究表明,Pt–MoO x / TiO 2的高活性归因于MoO x / TiO 2载体的Pt和路易斯酸位之间的协同作用。DOI:10.1002/cctc.201700219
-
作为产物:描述:十二烷醇 在 (bis[(2-diisopropylphosphino)ethyl]amine)Mn(CO)2 作用下, 反应 24.0h, 以76%的产率得到月桂醇月桂酸酯参考文献:名称:锰钳子配合物用于无碱,无受体的醇与酯的脱氢偶联反应:发展,范围和理解摘要:脂肪族PNP夹钳负载的地球上富集的锰(I)二羰基配合物可作为有效的催化剂,用于在无碱条件下将多种醇与酯进行无受体脱氢偶联。反应在纯净的条件下进行,催化剂负载适中,仅释放出H 2作为副产物。通过合成与催化循环有关的关键物质(通过X射线结构测定,多核(1 H,13 C,31 P,15 N,55Mn)NMR,红外光谱等),方法是研究与假定机理相关的基本步骤,并借助DFT计算。与相关的钌和铁PNP催化剂一样,脱氢是由PNP-Mn(CO)2支架的酰胺基和氨基氢化物形式之间的循环而引起的。对于将醇脱氢成醛,我们的结果表明,最高的能垒对应于氨基氢化物形式的氢释放,尽管其值接近于醇的外球脱氢成醛的值。这与钌和铁的催化体系形成鲜明对比,在钌和铁的催化体系中,与金属-配体协同作用中释放出的氢相比,将底物脱氢成醛的能源需求更少。DOI:10.1021/acscatal.6b03554
文献信息
-
Esterification of Aldehydes and Alcohols with Pyridinium Hydrobromide Perbromide in Water作者:Shinsei Sayama、Tetsuo OnamiDOI:10.1055/s-2004-835630日期:——The direct esterification of aldehydes and alcohols was carried out with pyridinium hydrobromide perbromide in water at room temperature. A variety of aldehydes were converted to respective ester derivatives with alcoholssuch as methanol, 1,2-ethanediol, 1,3-propanediol. Further, a variety of aliphatic alcohols were also converted to the corresponding Tishchenko-like dimeric esters in good yields under
-
Process for producing esters employing hydrolyzable catalysts申请人:Kendall Kirby J.公开号:US20050240040A1公开(公告)日:2005-10-27A process for producing esters wherein a carboxylic acid is reacted with an alcohol in the presence of a hydrolyzable catalyst in an aqueous medium under mixing to produce a reaction mixture comprising an organic phase containing ester and an aqueous phase.
-
Cerium or Ruthenium Catalyzed Oxidation of Alcohols to Carbonyl Compounds by Means of Sodium Bromate作者:Shigekazu Kanemoto、Hiroki Tomioka、Koichiro Oshima、Hitosi NozakiDOI:10.1246/bcsj.59.105日期:1986.1alcohols in the presence of cerium or ruthenium compounds in biphase reaction. Selective oxidation of secondary alcohols was performed in the presence of primary ones using cerium(IV) ammonium nitrate (CAN) or cerium(IV) sulfate (CS) as catalyst. For instance, treatment of 1,10-undecanediol with CS/NaBrO3 provided 11-hydroxy-2-undecanone in 82% yield. Ruthenium catalyzed biphase oxidation of alcohols
-
Oxidative dimerization of primary alcohols to esters catalyzed by iridium complexes作者:Aki Izumi、Yasushi Obora、Satoshi Sakaguchi、Yasutaka IshiiDOI:10.1016/j.tetlet.2006.10.144日期:2006.12Primary alcohols undergo efficiently oxidative dimerization by iridium complexes under air without any solvent to form esters in fair to good yields. For instance, the reaction of 1-dodecanol in the presence of [IrCl(coe)2]2 (3 mol %) at 95 °C for 15 h produced dodecyl dodecanoate in 91% isolated yield. This is the first successful Ir-catalyzed oxidative dimerization of primary alcohols to esters using
-
Synthesis of Unsymmetrical <i>N</i>-Heterocyclic Carbene–Nitrogen–Phosphine Chelated Ruthenium(II) Complexes and Their Reactivity in Acceptorless Dehydrogenative Coupling of Alcohols to Esters作者:Xiaochun He、Yaqiu Li、Haiyan Fu、Xueli Zheng、Hua Chen、Ruixiang Li、Xiaojun YuDOI:10.1021/acs.organomet.9b00071日期:2019.4.22dehydrogenative coupling of alcohols to esters, and the excellent isolated yields of esters were given in a catalyst loading of 1% for para- and meta-substituted benzyl alcohols and long-chain primary alcohols. Although some ortho-substituted benzyl alcohols displayed a relatively low reactivity due to the steric hindrance and the coordination of electron donor with the ruthenium center, the good product两种新型的钌络合物期RuH(CO)氯(PPH 3)(κ 2 -CP)(1)和[ FAC -RuH(CO)(PPH 3)(κ 3 -CNP)]氯(2)轴承不对称ñ -杂环合成了卡宾-氮-膦(CNP)并通过1 H NMR,31 P NMR和HRMS进行了表征。配合物2的结构通过单晶X射线衍射进一步确认。阴离子交换实验证明复合物2可以转化为复合物1在解决方案中。两种络合物在醇与酯的无受体脱氢偶联中显示出高催化性能,并且在对-和间-取代的苄醇和长链伯醇的催化剂负载量为1%时,酯的分离产率极佳。尽管由于空间位阻以及电子给体与钌中心的配位,一些邻位取代的苄醇显示出较低的反应性,但延长反应时间仍可获得良好的产物收率。特别地,该系统成功地实现了两种不同伯醇之间的酯的脱氢交叉偶联。
表征谱图
-
氢谱1HNMR
-
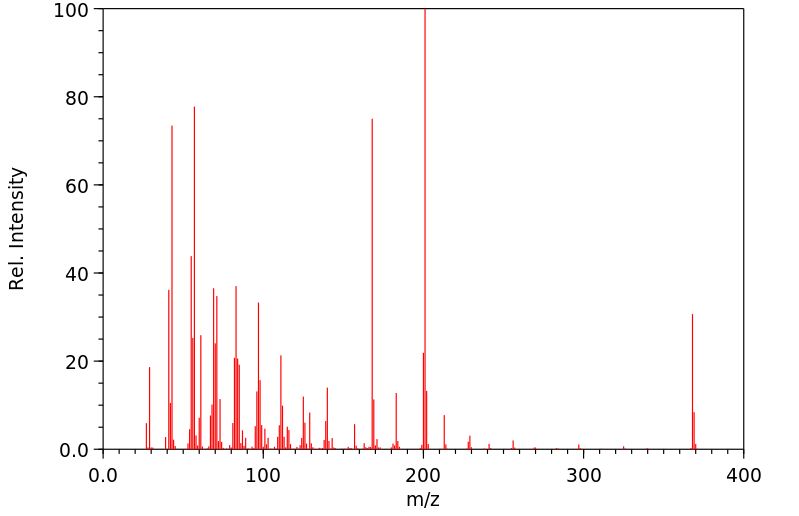
质谱MS
-
碳谱13CNMR
-
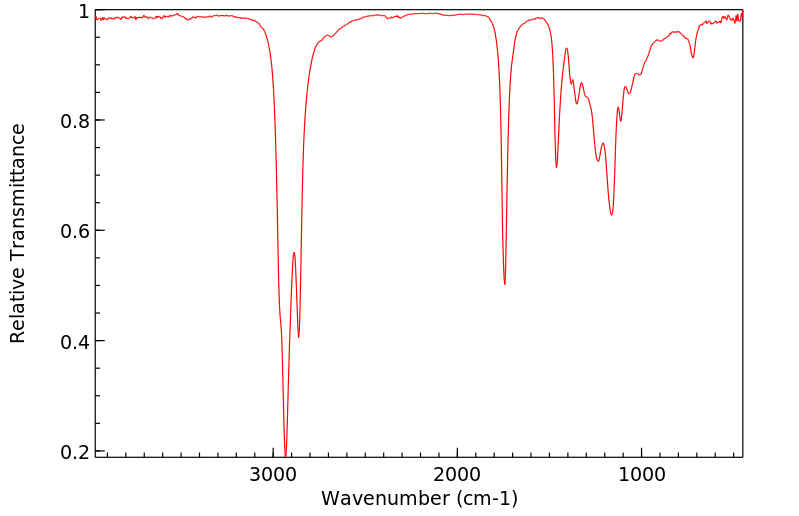
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯