蒎酮酸 | 473-72-3
中文名称
蒎酮酸
中文别名
——
英文名称
pinonic acid
英文别名
Pinonsaeure;2-(3-acetyl-2,2-dimethylcyclobutyl)acetic acid
CAS
473-72-3
化学式
C10H16O3
mdl
MFCD00066726
分子量
184.235
InChiKey
SIZDUQQDBXJXLQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:104~107℃
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:54.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
SDS
| Name: | cis-Pinonic acid 98% Material Safety Data Sheet |
| Synonym: | cis-3-Acetyl-2,2-dimethylcyclobutaneacetic aci |
| CAS: | 473-72-3 |
Synonym:cis-3-Acetyl-2,2-dimethylcyclobutaneacetic aci
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 473-72-3 | cis-Pinonic acid, 98% | 98% | 207-471-3 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
Causes gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower.
Exposure Limits CAS# 473-72-3: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear a chemical apron.
Respirators:
A NIOSH/MSHA approved air purifying dust or mist respirator or European Standard EN 149.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white to pale yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 104 - 107 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H16O3
Molecular Weight: 184.23
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 473-72-3: GU0200000 LD50/LC50:
Not available.
Carcinogenicity:
cis-Pinonic acid, 98% - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 473-72-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 473-72-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 473-72-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(3-乙酰基-2,2-二甲基环丁基)乙醛 (3-acetyl-2,2-dimethylcyclobutyl)acetaldehyde 2704-78-1 C10H16O2 168.236 2,6,6-三甲基双环[3.1.1]庚烷-3-酮 pinocamphone 18358-53-7 C10H16O 152.236 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-乙酰基-2,2-二甲基环丁烷乙酸甲酯 pinonic acid methyl ester 16978-11-3 C11H18O3 198.262 2-(3-乙酰基-2,2-二甲基环丁基)乙醛 (3-acetyl-2,2-dimethylcyclobutyl)acetaldehyde 2704-78-1 C10H16O2 168.236 蒎酸 pinic acid 28664-02-0 C9H14O4 186.208 6,6-二甲基二环[3.1.1]庚烷-2-酮 nopinone 24903-95-5 C9H14O 138.21 —— 2-(3-Acetyl-2,2-dimethylcyclobutyl)acetyl chloride 52557-22-9 C10H15ClO2 202.681 —— 3-(1,1-dimethyl-2-oxo-propyl)-glutaric acid 99974-72-8 C10H16O5 216.234 —— 1,1-Dimethyl-cyclobutancarbonsaeure-2-essigsaeure-4-diethylester 28664-03-1 C13H22O4 242.315
反应信息
-
作为反应物:描述:蒎酮酸 在 氢氧化钾 、 silver nitrate 作用下, 以 乙醚 为溶剂, 生成 3-(3-Acetyl-2,2-dimethyl-cyclobutyl)-propionic acid methyl ester参考文献:名称:Meikle,P.I.; Whittaker,D., Journal of the Chemical Society. Perkin transactions II, 1974, p. 318 - 321摘要:DOI:
-
作为产物:描述:参考文献:名称:溶液中亚硝基衍生物的光化学—XXX:温度对萜烯自由基加成反应和2-铵亚硝基链烷烃反应的影响摘要:研究了温度对铝自由基引发的光致加成反应的三个单萜的影响。N-亚硝基哌啶与樟脑和松烯的光加成反应,得到了α-哌啶叔亚硝基链烷烃,是由1,2-加成反应生成的主要加合物。烯的添加需要低温(−40°)才能抑制C自由基中间体的环丁烷开环途径。由于这些α-哌啶亚硝基链烷烃的官能团可呈现顺式-共面构型,因此它们容易发生裂解反应,得到相应的肟和铵盐。随着光解温度的升高,除松油外,环丁烷开环途径逐渐主导了反应,从而增加了8-亚硝基-p的含量。进行溶剂分解和消除反应的-薄荷烯衍生物。提出了一种机械解释,该解释支持C-自由基中间体的均相开环过程。结果建立了逐步添加到烯烃的诊断规则。追加了从商业sample烯样品中高效制备三环素的方法。DOI:10.1016/0040-4020(75)80237-7
文献信息
-
Identification and Quantification of Aerosol Polar Oxygenated Compounds Bearing Carboxylic or Hydroxyl Groups. 1. Method Development作者:M. Jaoui、T. E. Kleindienst、M. Lewandowski、E. O. EdneyDOI:10.1021/ac049919h日期:2004.8.1In this study, a new analytical technique was developed for the identification and quantification of multifunctional compounds containing simultaneously at least one hydroxyl or one carboxylic group, or both. This technique is based on derivatizing first the carboxylic group(s) of the multifunctional compound using an alcohol (e.g., methanol, 1-butanol) in the presence of a relatively strong Lewis acid (BF3) as a catalyst. This esterification reaction quickly and quantitatively converts carboxylic acids to their ester forms. The second step is based on silylation of the ester compounds using bis(trimethylsilyl) trifluoroacetamide (BSTFA) as the derivatizing agent. For compounds bearing ketone groups in addition to carboxylic and hydroxyl groups, a third step was used based on PFBHA derivatization of the carbonyls. Different parameters including temperature, reaction time, and effect due to artifacts were optimized. A GC/MS in EI and in methane-CI mode was used for the analysis of these compounds. The new approach was tested on a number of multifunctional compounds. The interpretation of their EI (70 eV) and CI mass spectra shows that critical information is gained leading to unambiguous identification of unknown compounds. For example, when derivatized only with BF3−methanol, their mass spectra comprise primary ions at m/z M•+ + 1, M•+ + 29, and M•+ − 31 for compounds bearing only carboxylic groups and M•+ + 1, M•+ + 29, M•+ − 31, and M+. − 17 for those bearing hydroxyl and carboxylic groups. However, when a second derivatization (BSTFA) was used, compounds bearing hydroxyl and carboxylic groups simultaneously show, in addition to the ions observed before, ions at m/z M•+ + 73, M•+ − 15, M•+ − 59, M•+ − 75, M•+ − 89, and 73. To the best of our knowledge, this technique describes systematically for the first time a method for identifying multifunctional oxygenated compounds containing simultaneously one or more hydroxyl and carboxylic acid groups.在本研究中,开发了一种新的分析技术,用于鉴定和定量含有至少一个羟基或一个羧基或两者的多官能团化合物。该技术基于首先使用醇(例如甲醇、1-丁醇)在相对较强的路易斯酸(BF3)催化下对多官能团化合物的羧基进行衍生化。这种酯化反应迅速且定量地将羧酸转化为酯形式。第二步是使用双(三甲基硅基)三氟乙酰胺(BSTFA)作为衍生化剂对酯化合物进行硅烷化。对于除羧基和羟基外还含有酮基的化合物,采用了基于PFBHA对羰基进行衍生化的第三步。优化了包括温度、反应时间和因人工产物引起的影响等多个参数。使用电子轰击源(EI)和甲烷化学电离(CI)模式的气相色谱/质谱法(GC/MS)分析了这些化合物。对多种多官能团化合物测试了新方法。对其EI(70 eV)和CI质谱的解释表明,获得了关键信息,从而能够明确鉴定未知化合物。例如,仅通过 −甲醇衍生化时,它们的质谱包含仅含有羧基的化合物的离子m/z M•+ + 1、M•+ + 29和M•+ − 31,以及同时含有羟基和羧基的化合物的离子m/z M•+ + 1、M•+ + 29、M•+ − 31和M•+ − 17。然而,当使用第二步衍生化(BSTFA)时,同时含有羟基和羧基的化合物除了之前观察到的离子外,还显示出m/z M•+ + 73、M•+ − 15、M•+ − 59、M•+ − 75、M•+ − 89和73的离子。据我们所知,这项技术首次系统地描述了一种鉴定同时含有一个或多个羟基和羧酸基团的多官能团含氧化合物的方法。
-
Antibiotic oxazolidinone derivatives申请人:Zeneca Ltd.公开号:US06365751B1公开(公告)日:2002-04-02The invention concerns a compound of the formula (I): wherein, for example: R1 is of the formula —NHC(═O)Rb wherein Rb is, for example, (1-4C)alkyl; R2 and R3 are hydrogen or fluoro; R2 and R3 are hydrogen or fluoro; D is O; R4 and R5 are hydrogen, (1-4C)alkyl or AR-oxymethyl; AR is phenyl or phenyl(1-4C)alkyl; R6 is hydrogen; >A—B— is of the formula >C═C(Ra)—, >CHCHRa—, or >C(OH)CHRa— (> represents two single bonds) wherein Ra is hydrogen or (1-4C)alkyl; and pharmaceutically-acceptable salts thereof; processes for their preparation; pharmaceutical compositions containing them and their use as antibacterial agents.本发明涉及如下公式(I)的化合物:其中,例如:R1是如下公式的形式—NHC(═O)Rb,其中Rb例如为(1-4C)烷基;R2和R3是氢或氟;D是氧;R4和R5是氢,(1-4C)烷基或AR-甲氧基甲基;AR是苯基或苯基(1-4C)烷基;R6是氢;>A—B—是如下公式的形式>C═C(Ra)—, >CHCHR_a—, 或 >C(OH)CHR_a—(>代表两个单键),其中Ra是氢或(1-4C)烷基;以及它们的药用可接受盐;它们的制备过程;含有它们的药物组合物以及它们作为抗菌剂的用途。
-
[EN] BICYCLIC IMIDAZOL DERIVATIVES AGAINST FLAVIVIRIDAE<br/>[FR] DERIVES DE L'IMIDAZOLE BICYCLIQUES DIRIGES CONTRE LES FLAVIVIRIDAE申请人:GENELABS TECH INC公开号:WO2005012288A1公开(公告)日:2005-02-10Disclosed are compounds, compositions and methods for treating Flaviviridae family virus infections.揭示了用于治疗黄病毒科病毒感染的化合物、组合物和方法。
-
Analysis of α-acyloxyhydroperoxy aldehydes with electrospray ionization-tandem mass spectrometry (ESI-MS<sup>n</sup>)作者:Bartłomiej Witkowski、Tomasz GierczakDOI:10.1002/jms.3130日期:2013.1α‐acyloxyhydroperoxy aldehydes. Ionization conditions were optimized. [M + H]+ ions were not formed in ESI; consequently, α‐acyloxyhydroperoxy aldehydes were identified as their ammonia adducts for the first time. On the other hand, atmospheric‐pressure chemical ionization has led to decomposition of the compounds of interest. Analysis of the mass spectra (MS2 and MS3) of the [M + NH4]+ ions allowed recognizing用直接注入电喷雾串联质谱(ESI / MS n)以及液相色谱和质谱(LC / MS)分析了一系列α-酰氧基氢过氧醛。α-酰氧基氢过氧醛的标准品是在羧酸存在下,通过环己烯的液相臭氧分解法制得的。稳定的Criegee中间体(SCI)是臭氧攻击环己烯双键的副产物,它与选定的羧酸(SCI清除剂)反应,导致形成α-酰氧基氢过氧醛。优化电离条件。[M + H] +ESI中没有形成离子;因此,首次将α-酰氧基氢过氧醛鉴定为其氨加成物。另一方面,大气压化学电离导致目标化合物的分解。[M + NH 4 ] +的质谱(MS 2和MS 3)分析离子可以识别所有研究化合物共有的碎片化途径。为了深入了解片段化机制,还研究了许多同位素标记的类似物。为了确认这种断裂机理可以预测不同的α-酰氧基氢过氧醛的质谱,对α-pine烯(一种非常重要的次要有机气溶胶前体)进行了臭氧分解。用α-ne烯,顺式制备的两种铵阳离子化的α-
-
Nickel-Catalyzed Decarboxylative Coupling of Redox-Active Esters with Aliphatic Aldehydes作者:Jichao Xiao、Zhenning Li、John MontgomeryDOI:10.1021/jacs.1c11170日期:2021.12.22The addition of alkyl fragments to aliphatic aldehydes is a highly desirable transformation for fragment couplings, yet existing methods come with operational challenges related to the basicity and instability of the nucleophilic reagents commonly employed. We report herein that nickel catalysis using a readily available bioxazoline (BiOx) ligand can catalyze the reductive coupling of redox-active
表征谱图
-
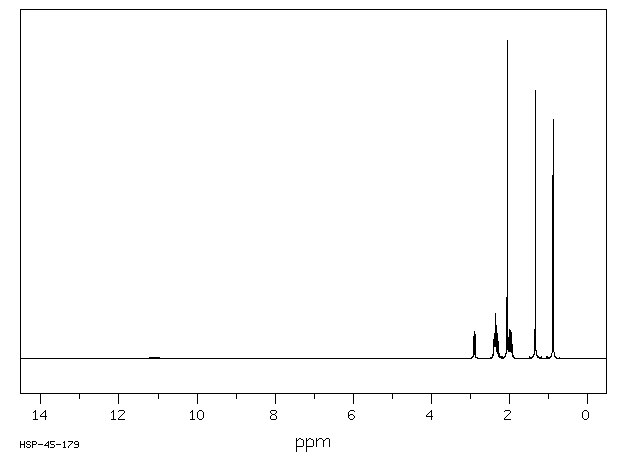
氢谱1HNMR
-
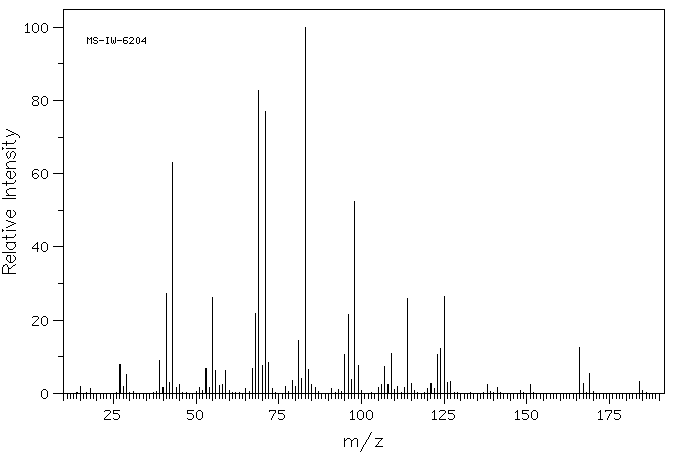
质谱MS
-
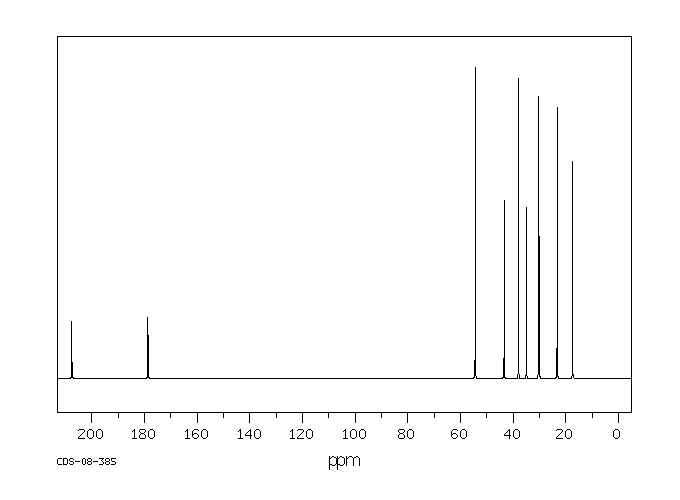
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷