2-甲基-5-己烯-3-醇 | 32815-70-6
中文名称
2-甲基-5-己烯-3-醇
中文别名
——
英文名称
2-methylhex-5-en-3-ol
英文别名
2-methyl-5-hexen-3-ol
CAS
32815-70-6
化学式
C7H14O
mdl
——
分子量
114.188
InChiKey
JKGMJZOZIJHPOH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:141.5-142 °C
-
密度:0.8459 g/cm3
-
LogP:1.780 (est)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:3
-
危险品运输编号:UN 1987
-
海关编码:2905290000
-
包装等级:III
-
安全说明:S16
-
危险类别码:R10
SDS
上下游信息
反应信息
-
作为反应物:描述:2-甲基-5-己烯-3-醇 在 sodium hydride 作用下, 以 四氢呋喃 、 乙醚 、 水 为溶剂, 生成 1-(1-isopropylbut-3-en-1-yloxy)but-3-en-2-one参考文献:名称:Synthesis of 3-oxooxa- and 3-oxoazacycloalk-4-enes by ring-closing metathesis. Application to the synthesis of an inhibitor of cathepsin K摘要:3-Oxooxa- and 3-oxoazacycloalk-4-enes were obtained with good yield from 1-(omega-alkenyloxy)- and 1-(omega-alkenylamino)-but-3-en-2-ones by using a ring-closing metathesis. This methodology has been used to synthesize an inhibitor of cathepsin K. (C) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2007.03.066
-
作为产物:描述:参考文献:名称:Nonplanar cyclobutane. Evidence for a conformationally controlled, classic mechanism in the deamination of cis- and trans-3-isopropylcyclobutylamine摘要:DOI:10.1021/jo01274a033
-
作为试剂:描述:1,3,5-三甲基己羟基-1,3,5-三嗪 在 2-甲基-5-己烯-3-醇 、 二氯二氟甲烷 、 2,4,5,6-四(9H-咔唑-9-基)异酞腈 、 叔丁基硫醇 作用下, 以 乙腈 为溶剂, 以98 %的产率得到1,3,5-trimethyl-2,3,4,5-tetrahydro-1,3,5-triazin-1-ium chloride参考文献:名称:缩醛胺作为强大的 XAT 试剂:氟化烷基氯的活化摘要:容易获得的 1,3,5-三甲基-1,3,5-三嗪烷可作为卤素原子转移的有效试剂。在光催化条件下,三嗪烷产生α-氨基烷基自由基,可以激活氟化烷基氯的C-Cl键。描述了氟化烷基氯和烯烃之间的氢氟烷基化反应。衍生自三嗪烷的二氨基取代基团的效率与立体电子效应有关,立体电子效应由六元环限定,迫使基团轨道和相邻氮原子的孤对进行反平面排列。DOI:10.1039/d3sc00027c
文献信息
-
The Exceptional Chelating Ability of Dimethylaluminum Chloride and Methylaluminum Dichloride. The Merged Stereochemical Impact of α- and β-Stereocenters in Chelate-Controlled Carbonyl Addition Reactions with Enolsilane and Hydride Nucleophiles作者:David A. Evans、Brett D. Allison、Michael G. Yang、Craig E. MasseDOI:10.1021/ja011337j日期:2001.11.1beta-disubstituted aldehydes concludes that the syn aldehyde diastereomer possesses the arrangement of stereocenters wherein the alpha- and beta-substituents impart a reinforcing facial bias upon the aldehyde carbonyl. Aldol reactions of syn aldehydes were thus observed to proceed with uniformly excellent diastereofacial selectivity. Aldol reactions of the corresponding anti aldehydes containing opposing
-
Palladium-Catalyzed Allylic Cross-Coupling Reactions of Primary and Secondary Homoallylic Electrophiles作者:Benjamin J. Stokes、Susanne M. Opra、Matthew S. SigmanDOI:10.1021/ja305403s日期:2012.7.18cross-coupling of homoallylic tosylate substrates using boronic acids and pinacol esters is reported. The reaction uses 2-(4,5-dihydro-2-oxazolyl)quinoline (quinox) as a ligand and is performed at ambient temperature. The scope of the reaction is broad in terms of both the boronate transmetalating reagent and the substrate and includes secondary tosylates. Mechanistic studies support an alkene-mediated
-
A highly efficient addition of allylic bromides to carbonyl compounds promoted by Cp2TiCl2(cat.)/Zn system作者:Yu Ding、Gang ZhaoDOI:10.1016/s0040-4039(00)74734-4日期:1992.12Aldehydes or ketones reacted with allylic bromides in the presence of Cp2TiCl2(cat.)/Zn system at room temperature to give homo-allylic alcohols in high yields.在室温下,在Cp 2 TiCl 2(cat。)/ Zn体系存在下,醛或酮与烯丙基溴反应,以高收率得到均烯丙基醇。
-
Water-Compatible Synthesis of 2-Trifluoromethyl-1,3-Dioxanes作者:Liliana Becerra-Figueroa、Alexander F. Tiniakos、Joëlle Prunet、Diego Gamba-SánchezDOI:10.1002/ejoc.201801367日期:2018.12.31A water-compatible method for the diastereoselective synthesis of 2-trifluormethyl-1,3-dioxanes is described. The reaction proceeds under mild reaction conditions using simple inorganic bases; it has a very good substrate scope and can be performed with different Michael acceptors. Additionally, the reaction products can be further functionalized, showing an excellent perspective for future applications
-
[EN] ANTIDIABETIC TRICYCLIC COMPOUNDS<br/>[FR] COMPOSÉS TRICYCLIQUES ANTIDIABÉTIQUES申请人:MERCK SHARP & DOHME公开号:WO2015051725A1公开(公告)日:2015-04-16Novel compounds of the structural formula (I), and the pharmaceutically acceptable salts thereof, are agonists of G-protein coupled receptor 40 (GPR40) and may be useful in the treatment, prevention and suppression of diseases mediated by the G-protein-coupled receptor 40. The compounds of the present invention may be useful in the treatment of Type 2 diabetes mellitus, and of conditions that are often associated with this disease, including obesity and lipid disorders, such as mixed or diabetic dyslipidemia, hyperlipidemia, hypercholesterolemia, and hypertriglyceridemia.
表征谱图
-
氢谱1HNMR
-
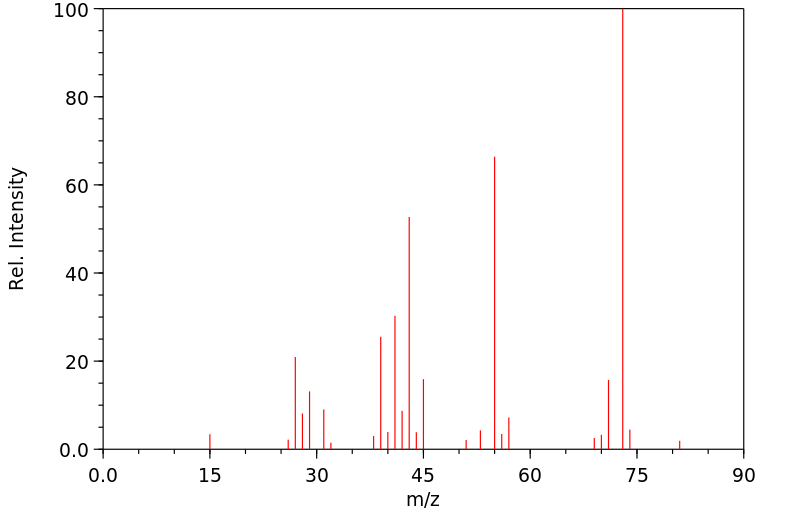
质谱MS
-
碳谱13CNMR
-
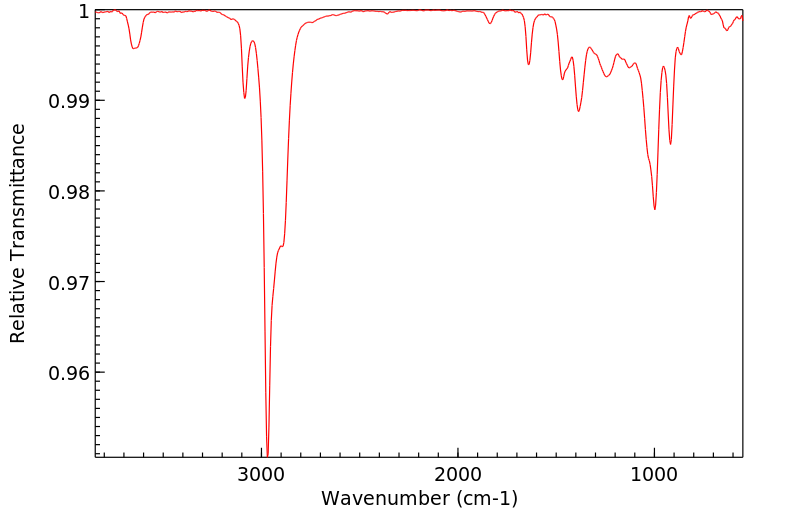
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷