顺式-2-丁烯-1-醇 | 4088-60-2
物质功能分类
中文名称
顺式-2-丁烯-1-醇
中文别名
——
英文名称
cis-2-buten-1-ol
英文别名
(Z)-2-buten-1-ol;(Z)-but-2-en-1-ol;(Z)-crotyl alcohol;cis-but-2-en-1-ol;2-Buten-1-ol
CAS
4088-60-2
化学式
C4H8O
mdl
——
分子量
72.1069
InChiKey
WCASXYBKJHWFMY-IHWYPQMZSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:37°C (estimate)
-
沸点:91.54°C (estimate)
-
密度:0.8532
-
LogP:0.636 (est)
-
溶解度:13.87 M
-
保留指数:596.5
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
SDS
上下游信息
反应信息
-
作为反应物:描述:顺式-2-丁烯-1-醇 在 pyridinium hydrobromide perbromide 作用下, 以 二氯甲烷 为溶剂, 反应 0.33h, 以77%的产率得到threo-2,3-Dibrombutan-1-ol参考文献:名称:Enantiopure Alleno-Acetylenic Cage Receptors对Danicalipin A甲基化和卤化片段的分子识别和共结晶摘要:Enantiopure (P)4- 和 (M)4-配置的aleno-炔笼 (AAC) 受体为立体化学复杂的氯硫脂 danicalipin A 的小分子片段的络合和结构解析提供了高度明确的内部结构。 溶液 (NMR),固体状态(X 射线)和对形成的主客体复合物的理论研究提供了对 danicalipin A 氯醇核心的 14 种非手性和手性衍生物的构象偏好的洞察,在封闭的、主要是疏水的环境中,扩展了先前报道的极性溶剂研究. 客体的保守结合模式允许破译官能团置换对吉布斯结合能 ΔG 的影响。揭示了构象能量对结合亲和力的强烈贡献,这解释了为什么客体较大的非极性域的密集堆积不一定会导致更高的关联。手性客体的对映选择性结合,非对映异构复合物之间的能量差异ΔΔG293 K高达0.7 kcal mol-1,可以通过氢键和卤素键以及分散相互作用来解释。量热研究 (ITC) 表明,一种对映异构体的更强结合伴随着焓DOI:10.1021/jacs.9b13217
-
作为产物:描述:参考文献:名称:Oxidative SIC bond cleavage of organotrifluorosilanes involving organic-group migration from hypercoordinate silicon to oxygen摘要:DOI:10.1016/s0040-4039(00)99364-x
-
作为试剂:描述:、 乙腈 在 顺式-2-丁烯-1-醇 、 silver carbonate 作用下, 反应 20.0h, 以14%的产率得到methyl (3S,4R)-3-(hydroxymethyl)-2-(2-methoxycarbonylphenyl)-4-methyl-3,4-dihydropyrazole-5-carboxylate参考文献:名称:Garanti, Luisa; Molteni, Giorgio; Zecchi, Gaetano, Heterocycles, 1995, vol. 40, # 2, p. 777 - 786摘要:DOI:
文献信息
-
Asymmetric Enzymatic Synthesis of Allylic Amines: A Sigmatropic Rearrangement Strategy作者:Christopher K. Prier、Todd K. Hyster、Christopher C. Farwell、Audrey Huang、Frances H. ArnoldDOI:10.1002/anie.201601056日期:2016.4.4Sigmatropic rearrangements, while rare in biology, offer opportunities for the efficient and selective synthesis of complex chemical motifs. A “P411” serine‐ligated variant of cytochrome P450BM3 has been engineered to initiate a sulfimidation/[2,3]‐sigmatropic rearrangement sequence in whole E. coli cells, a non‐natural function for any enzyme, providing access to enantioenriched, protected allylic amines
-
Palladium catalyzed C-allylation of nitroalkanes作者:Piotr Aleksandrowicz、Hanna Piotrowska、Wojciech SasDOI:10.1016/0040-4020(82)85120-x日期:1982.1study of palladium catalyzed C-allylation of aliphatic nitro compounds is described. Allylic alcohols and/or their acetates and allyl phenyl ethers were employed as allylating agents. The dependence of the reaction yield on nature and quantity of base and structure of the allylic unit is reported and explained. The lowest yield was obtained for allyl alcohol; however it could be considerably increased
-
Enantioselective Synthesis of α-Allyl Amino Esters via Hydrogen-Bond-Donor Catalysis作者:Andrew J. Bendelsmith、Seohyun Chris Kim、Masayuki Wasa、Stéphane P. Roche、Eric N. JacobsenDOI:10.1021/jacs.9b05556日期:2019.7.24diastereoselec-tive synthesis of α-allyl amino esters. The optimized protocol provides access to N-carbamoyl-protected amino esters via nucleophilic allyla-tion of readily accessible α-chloro glycinates. A variety of useful α-allyl amino esters were prepared-including crotylated products bearing vicinal stereocenters that are inaccessible through enolate alkylation-with high enantioselectivity (up to 97% ee)
-
Diastereoselective Ireland–Claisen rearrangements of substituted allyl β-amino esters: applications in the asymmetric synthesis of C(5)-substituted transpentacins作者:Stephen G. Davies、Ai M. Fletcher、James A. Lee、Paul M. Roberts、Myriam Y. Souleymanou、James E. Thomson、Charlotte M. ZammitDOI:10.1039/c4ob00274a日期:——The diastereoselective Ireland–Claisen rearrangement of a range of substituted allyl β-amino esters gave the corresponding enantiopure α-substituted-β-amino esters with good diastereoselectivity. The application of this methodology in the asymmetric synthesis of a range of C(5)-substituted 1,2-anti-1,5-syn-transpentacins was demonstrated by the rearrangement of a range of β-amino esters derived from
-
Metal complexes in organic synthesis—V作者:M. Moreno-Mañas、A. TriusDOI:10.1016/s0040-4020(01)98915-x日期:1981.1Pentane-2,4-dione with allylic alcohols or benzyl alcohol with palladium catalysts gives high yields of C-monoalkylated diketones, arising mainly from reaction at the terminal end of the allyic system for alkyl monosubstituted allyl alcohols. The effect of the catalyst on the alcohols has been evaluated; rearrangements and disproportionations have been observed.
表征谱图
-
氢谱1HNMR
-
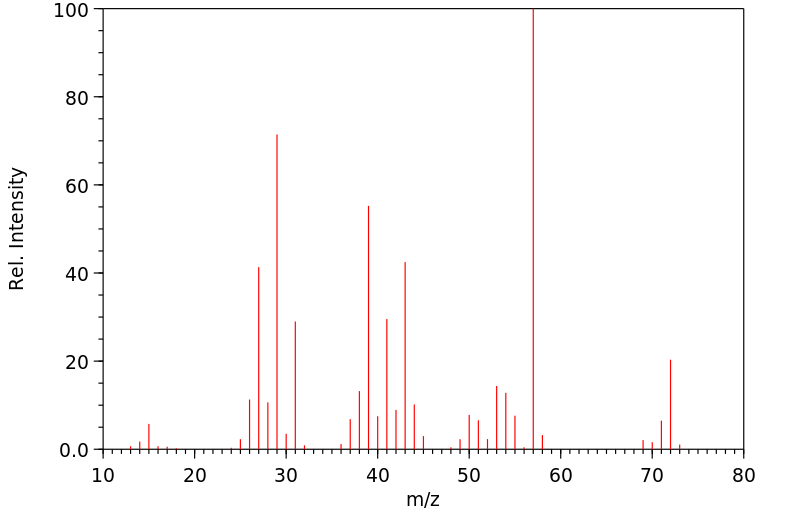
质谱MS
-
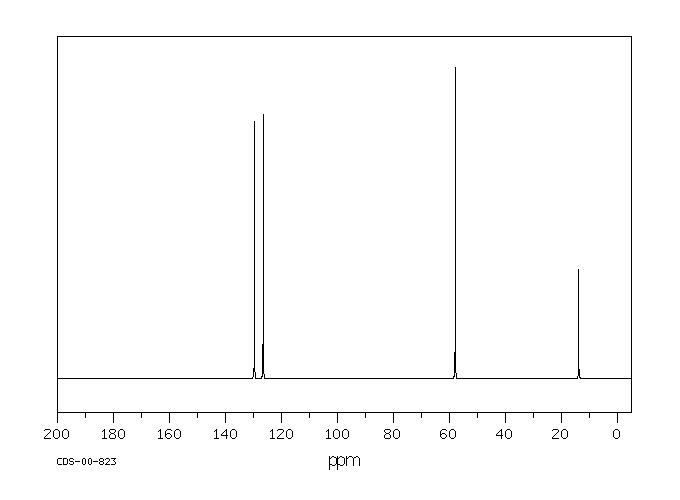
碳谱13CNMR
-
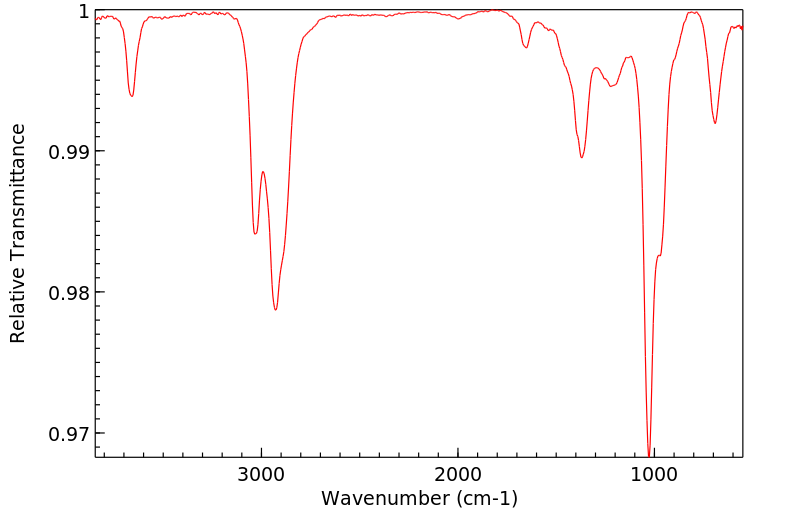
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷