乙基苯丙酮 | 16819-79-7
中文名称
乙基苯丙酮
中文别名
——
英文名称
1-(2-ethylphenyl)propan-1-one
英文别名
2-ethylpropiophenone;2'-Ethylpropiophenone
CAS
16819-79-7
化学式
C11H14O
mdl
——
分子量
162.232
InChiKey
RESNKGBFZAPADB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:250.2±19.0 °C(Predicted)
-
密度:0.98
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H312,H315,H319,H332,H335
反应信息
-
作为反应物:参考文献:名称:外消旋α-溴酮与芳基锌试剂的催化不对称交叉偶联摘要:镍盒:已开发出第一种用于将芳基锌试剂与 α-溴酮交叉偶联的催化不对称方法(参见方案)。这种立体会聚的碳-碳键形成过程发生在异常温和的条件下并且没有活化剂,从而允许产生潜在的不稳定的三级立体中心。DOI:10.1002/anie.200804888
-
作为产物:描述:参考文献:名称:一种盐酸乙哌立松杂质F的制备方法摘要:本发明提供一种盐酸乙哌立松杂质F的制备方法,以2‑溴乙苯为原料,通过格氏反应得到1‑(2‑乙基苯基)丙醇,再通过氧化反应得到2‑乙基苯丙酮,最后2‑乙基苯丙酮和多聚甲醛、哌啶盐酸盐一锅法经过曼尼希反应得到盐酸乙哌立松杂质F,盐酸乙哌立松杂质F结构如式(Ⅰ)所示,采用本发明提供的技术方案所得盐酸乙哌立松杂质F纯度可达99%,可作为对照品用于实验研究。公开号:CN112390767A
文献信息
-
Regioselectivity of the Base-Induced Ring Cleavage of 1-Oxygenated Derivatives of Cyclobutabenzene作者:Abha Gokhale、Peter SchiessDOI:10.1002/hlca.19980810207日期:1998.2.4products through distal and/or proximal cleavage of the strained four-membered ring via benzyl carbanion 4 and/or aryl carbanion 5. A systematic study of this process reveals the relative stability of the two isomeric carbanions 4 and 5 as a key factor in determining the course of the ring-cleavage reaction. While benzyl carbanions 4 can be trapped with carbon electrophiles, attempts at trapping aryl氧基阴离子3从1,2- dihydrocyclobutabenzen -1-酮生成1通过添加带电荷的亲核试剂的或由1-羟基-1,2- dihydrocyclobutabenzenes 2通过去质子化用碱导致稳定的产品通过远端和/或近端的裂解通过苄基碳负离子4和/或芳基碳负离子5拉紧四元环。对这一过程的系统研究表明,两个同分异构碳负离子4和5的相对稳定性是决定环断裂反应过程的关键因素。而苄基碳负离子4可以被碳亲电试剂捕获,尝试用非H +亲电试剂捕获芳基碳负离子5失败。在质子溶剂中,叔醇2的镁盐与其碱金属盐相比,具有更高的近端裂解速率。由此,我们得出结论,与苄基碳负离子4相比,游离芳基碳负离子5仅是瞬时存在的。近侧C,C-键裂解似乎通过质子化或者发生5从快速,可逆平衡3 ⇌ 5,其中3甚至在质子溶剂中可能占主导地位,甚至可能在芳香族C原子处通过限速质子化3来绕过游离阴离子5。因此,在确定酮1和
-
METHOD FOR PRODUCING CYCLOHEXYL ALKYL KETONES申请人:Nishiuchi Junya公开号:US20120178970A1公开(公告)日:2012-07-12Provided is an industrially superior method for producing cyclohexyl alkyl ketones, which solves the problems in process reduction and in disposal of wastes such as metals. An aromatic ketone represented by a formula (1) is nuclear-hydrogenated with pressurized hydrogen and in the presence of a solvent at a temperature of from 20 to 120° C., in the presence of a catalyst that carries from 0.1 to 20% by weight of a ruthenium atom on the carrier, thereby producing a cyclohexyl alkyl ketone represented by a formula (2): provided that, in the formula (2), n indicates an integer of from 1 to 3; R represents a hydroxyl group, a cyclohexyl group, an alkyl group having from 1 to 4 carbon atoms, or an acyl group having from 1 to 4 carbon atoms
-
PROCESS FOR PREPARATION OF NORBORNENE DERIVATIVES申请人:JX Nippon Oil & Energy Corporation公开号:EP2444386A1公开(公告)日:2012-04-25A method for producing a norbornene derivative, comprising: a first step of forming a Mannich base by reacting a carbonyl compound and an amine compound with each other in an acidic solvent, to thereby obtain a reaction liquid comprising the Mannich base in the acidic solvent, the acidic solvent comprising a formaldehyde derivative and 0.01 mol/L or more of an acid represented by the formula: HX (In the formula, X represents F or the like), the carbonyl compound being represented by any of the following general formulae (1) to (3): [in formulae (1) to (3), R1, R2, R3, R4, R5, and R6 each independently represent a hydrogen atom or the like, and n represents an integer of any of 0 to 4], the amine compound being represented by the following general formula (4): [in the formula (4), R7S each independently represent a linear chain saturated hydrocarbon group having 1 to 20 carbon atoms or the like, and X- represents F- or the like], the Mannich base being represented by any of the following general formulae (5) to (7): [R1, R2, R3, R4, R5, R6, and n in the formulae (5) to (7) have the same meanings as those of R1, R2, R3, R4, R5, R6, and n in the formulae (1) to (3), and R7 and X- in the formulae (5) to (7) have the same meanings as those of R7 and X- in the formula (4)] and a second step of reacting the Mannich base and a diene compound with each other by adding an organic solvent, a base in an amount of 1.0 to 20.0 equivalents to the acid, and the diene compound to the reaction liquid, and then heating the reaction liquid, to thereby form a norbornene derivative, the diene compound being represented by the following general formula (8): [in the formula (8), R8 represents a hydrogen atom or the like], the norbornene derivative being represented by any of the following general formulae (9) to (11): [R1, R2, R3, R4, R5, R6, and n in the formulae (9) to (11) have the same meanings as those of R1, R2, R3, R4, R5, R6, and n in the formulae (1) to (3), and R8 in the formulae (9) to (11) has the same meaning as that of R8 in the formula (8)].一种制备去氢莰烯衍生物的方法,包括以下步骤:第一步,在酸性溶剂中使羰基化合物和胺基化合物反应,形成曼尼希碱,从而在酸性溶剂中获得包含曼尼希碱的反应液,所述酸性溶剂包括甲醛衍生物和代表为HX的酸,其中HX中的X代表F或类似物,所述羰基化合物由以下通式(1)至(3)中的任一通式表示:[在通式(1)至(3)中,R1、R2、R3、R4、R5和R6各自独立地代表氢原子或类似物,n代表0至4中的任一整数],所述胺基化合物由以下通式(4)表示:[在通式(4)中,R7S各自独立地代表具有1至20个碳原子或类似物的线性链饱和碳氢基团,X-代表F-或类似物],所述曼尼希碱由以下通式(5)至(7)中的任一通式表示:[在通式(5)至(7)中,R1、R2、R3、R4、R5、R6和n的含义与通式(1)至(3)中的R1、R2、R3、R4、R5、R6和n的含义相同,通式(5)至(7)中的R7和X-的含义与通式(4)中的R7和X-的含义相同];第二步,通过向反应液中加入有机溶剂、相当于酸的1.0至20.0当量的碱和二烯化合物,然后加热反应液,使曼尼希碱与二烯化合物发生反应,从而形成去氢莰烯衍生物,所述二烯化合物由以下通式(8)表示:[在通式(8)中,R8代表氢原子或类似物],所述去氢莰烯衍生物由以下通式(9)至(11)中的任一通式表示:[在通式(9)至(11)中,R1、R2、R3、R4、R5、R6和n的含义与通式(1)至(3)中的R1、R2、R3、R4、R5、R6和n的含义相同,通式(9)至(11)中的R8的含义与通式(8)中的R8的含义相同]。
-
ORGANIC COMPOUNDS AND THEIR USES申请人:Brandl Trixl公开号:US20100204159A1公开(公告)日:2010-08-12The present application describes organic compounds that are useful for the treatment, prevention and/or amelioration of human diseases.本申请描述了对人类疾病的治疗、预防和/或改善有用的有机化合物。
-
An efficient MnCl<sub>2</sub>-catalyzed tandem acylation-cross-coupling reaction of<i>o</i>-halobenzoyl chloride with diorganyl magnesium compounds作者:Fengmin Zhang、Zhuangzhi Shi、Feng Chen、Yu YuanDOI:10.1002/aoc.1567日期:——An efficient tandem cross‐coupling reaction of o‐chlorobenzoyl chloride with dialkyl and diaryl magnesium compounds in the presence of manganese (II) chloride was developed, which proceeds in good yield under mild conditions. Copyright © 2009 John Wiley & Sons, Ltd.
表征谱图
-
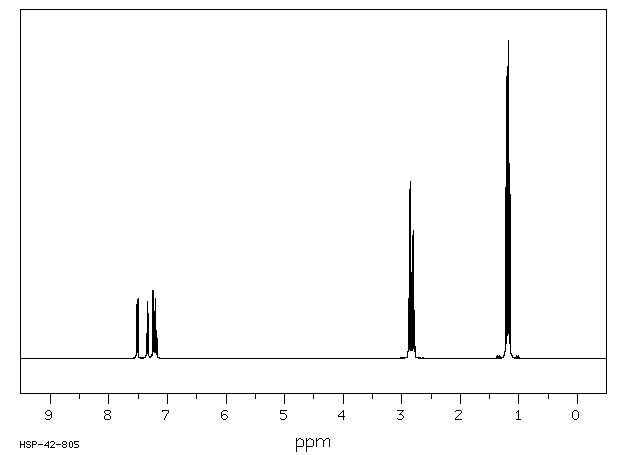
氢谱1HNMR
-
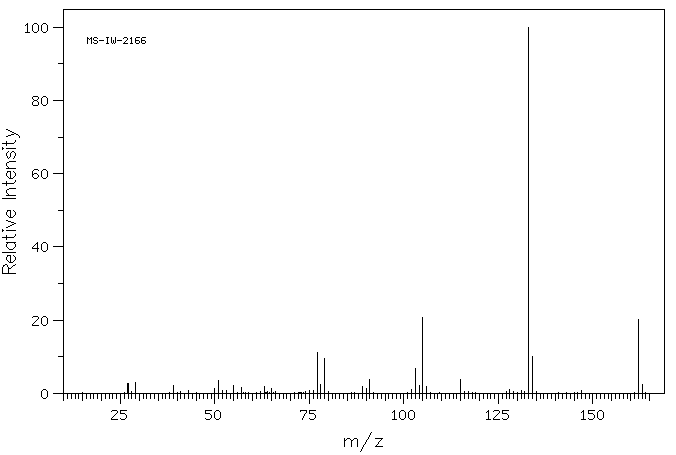
质谱MS
-
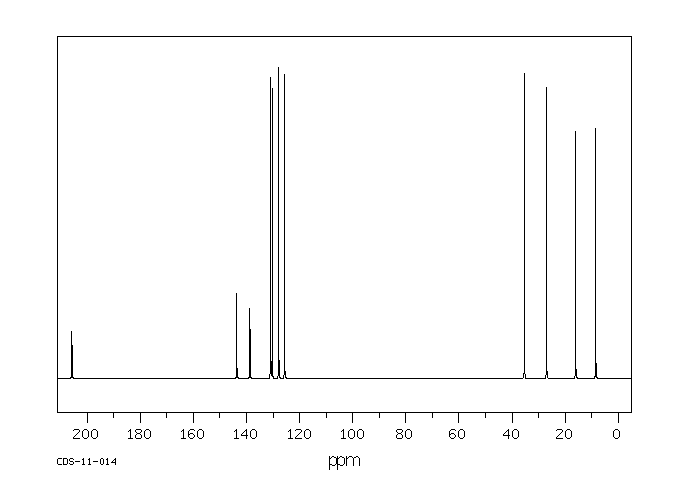
碳谱13CNMR
-
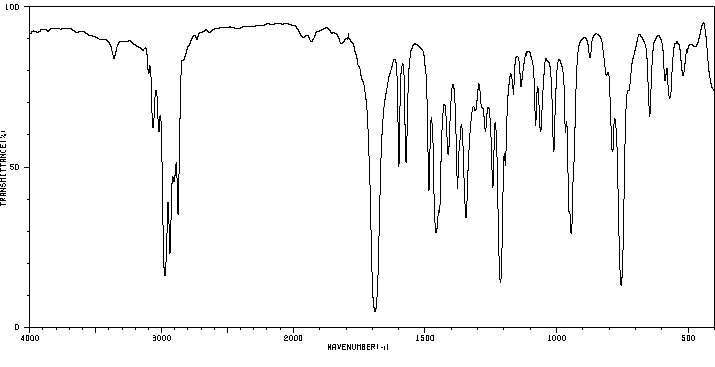
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷