1-(4-碘苯基)吡咯 | 92636-36-7
中文名称
1-(4-碘苯基)吡咯
中文别名
1-(4-碘苯基)吡咯;1-(4-碘苯基)吡 咯
英文名称
1-(4-iodophenyl)pyrrole
英文别名
1-(4-iodophenyl)-1H-pyrrole
CAS
92636-36-7
化学式
C10H8IN
mdl
MFCD00052399
分子量
269.085
InChiKey
FMURNAZHVQDQQN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:128-133 °C
-
沸点:302.0±25.0 °C(Predicted)
-
密度:1.6897 (estimate)
-
稳定性/保质期:
按规定使用和贮存的情况下不会分解,应避开氧化物、光线。
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:4.9
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
安全说明:S22,S24/25,S26,S36/37/39
-
储存条件:请将药品存放在密闭、阴凉、干燥的地方。
SDS
| Name: | 1-(4-Iodophenyl)pyrrole Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 92636-36-7 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 92636-36-7 | 1-(4-Iodophenyl)pyrrole | 98 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Light sensitive.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Store protected from light.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store protected from light.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 92636-36-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 131 - 133 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H8IN
Molecular Weight: 268.9672
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable. Light sensitive.
Conditions to Avoid:
Light, dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents, acid chlorides, acid anhydrides, magnesium.
Hazardous Decomposition Products:
Carbon monoxide, oxides of phosphorus, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 92636-36-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(4-Iodophenyl)pyrrole - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 92636-36-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 92636-36-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 92636-36-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苯基吡咯 1-phenylpyrrole 635-90-5 C10H9N 143.188 4-(1-吡咯)苯酚 4-(1H-pyrrol-1-yl)phenol 23351-09-9 C10H9NO 159.188 4-(1H-吡咯-1-基)苯甲醛 4-(1H-pyrrol-1-yl)benzaldehyde 23351-05-5 C11H9NO 171.199 4-(1H-吡咯-1-基)苯甲腈 4-(1H-pyrrol-1-yl)benzonitrile 23351-07-7 C11H8N2 168.198 —— 1-(4-(phenylselenide)phenyl)-1H-pyrrole —— C16H13NSe 298.246 1-[4-(三氟甲基)苯基]-1H-吡咯 1-(4-(trifluoromethyl)phenyl)-1H-pyrrole 92636-38-9 C11H8F3N 211.186
反应信息
-
作为反应物:描述:1-(4-碘苯基)吡咯 在 potassium nitrite 、 copper(II) bis(trifluoromethanesulfonate) 作用下, 以 二甲基亚砜 为溶剂, 反应 48.0h, 以78%的产率得到1-(4-硝基苯基)-1H-吡咯参考文献:名称:铜催化本位无配体的条件下iodoarenes,bromoarenes和杂环卤代芳烃的-nitration摘要:在无配体的条件下使用铜盐提出了将卤代芳烃转化为相应的硝基芳烃的催化方案。该方法有效地用于本位的各种各样的卤代芳烃包括iodoarenes,bromoarenes和杂环卤代芳烃-nitration。DOI:10.1016/j.tetlet.2012.01.056
-
作为产物:描述:参考文献:名称:Iron-Catalyzed Inexpensive and Practical Synthesis of N-Substituted Pyrroles in Water摘要:一种操作简便、实用且经济的实验方案已被开发,用于在水相中以铁(III)氯化物为催化剂进行Paal-Knorr吡咯合成,产率良好至优异。在非常温和的反应条件下,多个N-取代吡咯可以方便地通过2,5-二甲氧基四氢呋喃与芳基/烷基、磺酰基和酰基胺的反应来制备。DOI:10.1055/s-0029-1217799
文献信息
-
Water-Sculpting of a Heterogeneous Nanoparticle Precatalyst for Mizoroki–Heck Couplings under Aqueous Micellar Catalysis Conditions作者:Haobo Pang、Yuting Hu、Julie Yu、Fabrice Gallou、Bruce H. LipshutzDOI:10.1021/jacs.0c11484日期:2021.3.10Powdery, spherical nanoparticles (NPs) containing ppm levels of palladium ligated by t-Bu3P, derived from FeCl3, upon simple exposure to water undergo a remarkable alteration in their morphology leading to nanorods that catalyze Mizoroki–Heck (MH) couplings. Such NP alteration is general, shown to occur with three unrelated phosphine ligand-containing NPs. Each catalyst has been studied using X-ray粉末状球形纳米粒子 (NPS) 含有 ppm 级的钯,由来自 FeCl 3的t- Bu 3 P连接,在简单暴露于水中后,其形态发生显着变化,导致纳米棒催化 Mizoroki-Heck (MH) 偶联。这种 NP 改变是普遍的,显示发生在三个不相关的含膦配体的 NP 中。已使用 X 射线光电子能谱 (XPS)、能量色散 X 射线能谱 (EDX)、透射电子显微镜 (TEM) 和低温透射电子显微镜 (cryo-TEM) 分析研究了每种催化剂。专门依赖于含有t- Bu 3 的NPS 的联轴器P-连接的 Pd 在室温至 45 °C 之间的水性胶束催化条件下发生,并显示出广泛的底物范围。与这项新技术相关的其他关键特性包括产品中残留的 Pd 低、水性反应介质的循环利用以及相关的低 E 因子。galipinine 是汉考克生物碱家族的一员,其前体的合成暗示了潜在的工业应用。
-
Squaric acid catalyzed simple synthesis of N-substituted pyrroles in green reaction media作者:Najmadin Azizi、Anahita Davoudpour、Farshid Eskandari、Ehlham BatebiDOI:10.1007/s00706-012-0841-2日期:2013.3AbstractAn operationally simple and efficient protocol for squaric acid catalyzed synthesis of N-substituted pyrroles via the reaction of 2,5-dimethoxytetrahydrofuran and 2,5-hexandione with aryl amines in green reaction media (water, deep eutectic solvent, and polyethylene glycol) under ultrasound irradiation or thermal conditions in good to excellent yields has been developed. Graphical abstract
-
Reactive organoallyl species generated from aryl halides and allene: allylation of α,β-unsaturated aldehydes and cyclic ketones employing Pd/In transmetallation processes作者:Laura A.T. Cleghorn、Ronald Grigg、Vladimir Savic、Milena SimicDOI:10.1016/j.tet.2008.06.100日期:2008.9β-unsaturated aldehydes and cyclic ketones promoted by Pd/In transmetallation processes has been studied. The unsaturated aldehydes underwent regioselective 1,2-addition to afford secondary homoally alcohols. The reactions have been performed using Pd(OAc)2/PPh3 as catalytic system and metallic indium affording the products in good yields. The same transformation with unsaturated ketones proved to be less efficient
-
GaCl<sub>3</sub>-Catalyzed C–H Cyanation of Indoles with <i>N</i>-Cyanosuccinimide作者:Xue Wang、Mohamed Makha、Shu-Wei Chen、Huaiji Zheng、Yuehui LiDOI:10.1021/acs.joc.9b00416日期:2019.5.17An efficient GaCl3-catalyzed direct cyanation of indoles and pyrroles using bench-stable electrophilic cyanating agent N-cyanosuccinimide was achieved and afforded 3-cyanoindoles and 2-cyanopyrroles in good yields and excellent regioselectivities. Notably, this protocol exhibited high reactivity for unprotected indoles and was applicable to a broad range of indole and pyrrole substrates.
-
Halogen-Bond-Promoted Photoactivation of Perfluoroalkyl Iodides: A Photochemical Protocol for Perfluoroalkylation Reactions作者:Yaxin Wang、Junhua Wang、Guo-Xing Li、Gang He、Gong ChenDOI:10.1021/acs.orglett.7b00375日期:2017.3.17irradiation from a compact fluorescent lamp, low-intensity UV lamp, or sunlight. This protocol can be applied to the synthesis of perfluoroalkyl-substituted phenanthridines as well as effect the iodo-perfluoroalkylation of alkenes/alkynes and the C–H perfluoroalkylation of electron-rich arenes and heteroarenes. This C–H perfluoroalkylation reaction offers a unique method for site-selective labeling of oligopeptides
表征谱图
-
氢谱1HNMR
-
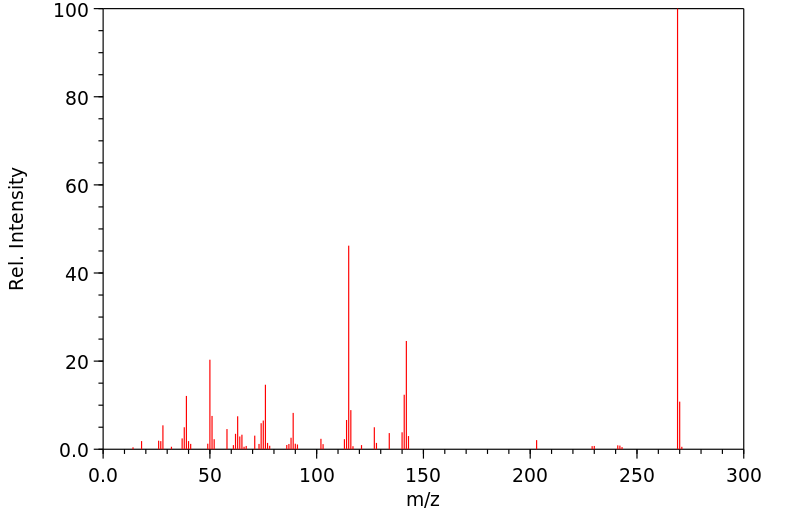
质谱MS
-
碳谱13CNMR
-
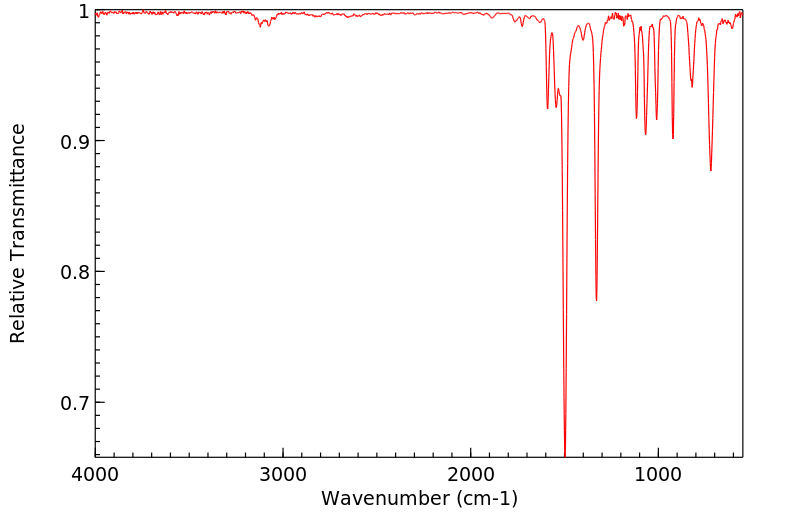
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄胆红酸
高树蛙毒素
颜料红2254
阿根诺卡菌素
阿托伐他汀镁
阿托伐他汀钙阿托伐他汀钙中间体1甲酯
阿托伐他汀钙杂质59
阿托伐他汀钙杂质52
阿托伐他汀钙杂质43
阿托伐他汀钙杂质
阿托伐他汀钙杂质
阿托伐他汀钙三水合物
阿托伐他汀钙L-8
阿托伐他汀钙
阿托伐他汀酸异丙酯
阿托伐他汀酰基-Β-D-葡糖苷酸
阿托伐他汀缩丙酮
阿托伐他汀相关化合物E
阿托伐他汀甲酯
阿托伐他汀甲胺盐
阿托伐他汀烯丙基酯
阿托伐他汀杂质F
阿托伐他汀杂质95
阿托伐他汀杂质5
阿托伐他汀杂质31
阿托伐他汀杂质1
阿托伐他汀叔丁酯
阿托伐他汀双氟杂质中间体
阿托伐他汀内酯-[D5]
阿托伐他汀内酯
阿托伐他汀乙酯
阿托伐他汀USP相关物质E
阿托伐他汀L1二胺物杂质
阿托伐他汀3-羟基消除杂质
阿托伐他汀3-氧杂质
阿托伐他汀
阿利考昔
阿伐他汀钠
镍(II)(吡唑二氰胺)2
镉原卟啉IX二甲酯
铬,二溴二(吡啶)-
达考帕泛
费耐力
角质形成细胞分化诱导剂
西拉美新盐酸盐
西拉美新
虫螨腈
萨格列扎
苏尼替尼N-1
芬度柳