4,4'-二氯二苯甲醇 | 90-97-1
中文名称
4,4'-二氯二苯甲醇
中文别名
4,4'-二氯二苯基甲醇;4,4’-二氯二苯基甲醇;4,4"-二氯二苯甲醇
英文名称
4,4'-dichlorobenzophenone
英文别名
bis(4-chlorophenyl)methanol;4,4'-dichlorobenzhydrol;4,4′-dichlorobenzhydrol;4,4’-dichlorobenzhydrol
CAS
90-97-1
化学式
C13H10Cl2O
mdl
MFCD00000629
分子量
253.128
InChiKey
PHUYGURFBULKPA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:91-95 °C
-
沸点:386.1±32.0 °C(Predicted)
-
密度:1.2806 (estimate)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.076
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
危险类别码:R22,R36/37/38
-
海关编码:2906299090
-
安全说明:S26,S37/39
-
储存条件:本品应密封并存放在阴凉处。
SDS
4,4'-二氯二苯甲醇 修改号码:5
模块 1. 化学品
产品名称: 4,4'-Dichlorobenzhydrol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二氯二苯甲醇
百分比: >98.0%(GC)
CAS编码: 90-97-1
分子式: C13H10Cl2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
4,4'-二氯二苯甲醇 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
93°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
4,4'-二氯二苯甲醇 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
4,4'-二氯二苯甲醇 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4,4'-Dichlorobenzhydrol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二氯二苯甲醇
百分比: >98.0%(GC)
CAS编码: 90-97-1
分子式: C13H10Cl2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
4,4'-二氯二苯甲醇 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
93°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
4,4'-二氯二苯甲醇 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
4,4'-二氯二苯甲醇 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
制备方法
有机合成。
用途简介 用途有机合成。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-[bromo(4-chlorophenyl)methyl]-4-chlorobenzene 6306-46-3 C13H9BrCl2 316.024 4,4'-二氯二苯甲酮 4,4'-Dichlorobenzophenone 90-98-2 C13H8Cl2O 251.112 1-氯-4-[氯(4-氯苯基)甲基]苯 chlorobis(4-chlorophenyl)methane 782-08-1 C13H9Cl3 271.573 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,1'-(甲氧基亚甲基)二(4-氯苯) 1,1-bis(4-chlorophenyl)-methoxymethane 55702-41-5 C14H12Cl2O 267.155 1-氯-4-[(4-氯苯基)-乙氧基甲基]苯 4,4'-dichlorobenzhydryl ethyl ether 57070-99-2 C15H14Cl2O 281.182 二苯甲醇 1,1-Diphenylmethanol 91-01-0 C13H12O 184.238 1-[二(4-氯苯基)甲氧基-(4-氯苯基)甲基]-4-氯苯 bis-4,4'-dichlorobenzhydryl ether 74562-99-5 C26H18Cl4O 488.24 —— 2-(bis(4-chlorophenyl)methoxy)ethyl chloride 5409-90-5 C15H13Cl3O 315.627 —— 1-bromo-2-[bis(4-chlorophenyl)methoxy]ethane 221916-05-8 C15H13BrCl2O 360.078 1,1’-亚甲基双(4-氯苯) 4,4'-dichlorodiphenylmethane 101-76-8 C13H10Cl2 237.128 —— 4,4'-Dichlorobenzhydryl acetate 285130-02-1 C15H12Cl2O2 295.165 三(4-氯苯基)甲烷 tris(4-chlorophenyl)methane 27575-78-6 C19H13Cl3 347.671 —— bis-(4-chloro-phenyl)-phenyl-methane 80428-22-4 C19H14Cl2 313.226 2-[双(4-氯苯基)甲氧基]-N,N-二甲基乙酰胺 2-[Bis-(4-chloro-phenyl)-methoxy]-N,N-dimethyl-acetamide 41858-32-6 C17H17Cl2NO2 338.233 —— 1,1-bis(4-chlorophenyl)prop-2-en-1-ol 86147-03-7 C15H12Cl2O 279.166 —— 1-{2-[Bis-(4-chloro-phenyl)-methoxy]-ethyl}-piperidine 143747-57-3 C20H23Cl2NO 364.315 —— Oxalsaeure-di-<4,4'-dichlor-benzhydrylester> 16642-43-6 C28H18Cl4O4 560.261 —— 1-[bromo(4-chlorophenyl)methyl]-4-chlorobenzene 6306-46-3 C13H9BrCl2 316.024 4,4'-二氯二苯甲酮 4,4'-Dichlorobenzophenone 90-98-2 C13H8Cl2O 251.112 4-氯-alpha-(4-氯苯基)苯甲胺 bis(4-chlorophenyl)methanamine 14212-38-5 C13H11Cl2N 252.143 1-氯-4-[氯(4-氯苯基)甲基]苯 chlorobis(4-chlorophenyl)methane 782-08-1 C13H9Cl3 271.573 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:一种芳香腈或杂芳香腈类化合物的制备方法摘要:本发明公开了一种芳香腈或杂芳香腈类化合物的制备方法,其包括下述的步骤:在惰性气体保护下,溶剂中,在镍催化剂及配体,金属锌,及添加剂作用下,将氰基化试剂与卤代芳烃或卤代杂芳烃反应即可。本发明提供的制备方法,使用廉价易得的镍催化剂和配体,能温和、高效地实现将卤代芳烃或卤代杂芳烃特别是能将廉价易得但反应低活性的氯代芳烃或氯代杂芳烃与毒性较小的氰基化试剂反应,制备得到芳香腈或杂芳香腈类化合物。本发明的制备方法不仅具有操作简捷、温和、高效等优点,而且还具有良好的官能团兼容性和底物普适性等特征。公开号:CN108623495A
-
作为产物:描述:4,4'-二氯二苯甲酮 在 sodium tetrahydroborate 作用下, 以 甲醇 为溶剂, 以100%的产率得到4,4'-二氯二苯甲醇参考文献:名称:结构新颖的 1,3-二取代氮杂环丁烷衍生物的便捷合成摘要:摘要描述了结构新颖的 1,3-二取代氮杂环丁烷衍生物的方便合成。该方法涉及将氮杂环丁烷结构单元与磺酰化氨基甲酸甲酯缩合,随后用胺猝灭亚氨酰氯。通过取代二苯甲醇和苯磺酰胺作为核心结构的一部分来制备不同的衍生物。DOI:10.1080/00397910801982340
-
作为试剂:参考文献:名称:在氧气气氛下使用氢化钠通过将苯氢酚氧化为二苯甲酮而原位生成氢过氧化物:用于将环状1,2-二酮氧化裂解为二羧酸摘要:已经开发了用现场产生的氢过氧化物容易地将环状1,2-二酮1氧化为二羧酸3的方法。通过在氧气气氛下使用氢化钠将4,4'-二氯苯甲酸2f氧化为4,4'-二氯二苯甲酮4f进行氢过氧化物的原位生成。DOI:10.1016/j.tetlet.2012.10.127
文献信息
-
Waste-Free Swift Synthesis of Symmetrical and Unsymmetrical Diarylmethyl Thioethers from Diaryl Carbinols作者:Rama Peddinti、Pallavi SinghDOI:10.1055/s-0036-1589022日期:2017.8to synthesize symmetrical and unsymmetrical diarylmethyl thioethers from diaryl carbinols and thiols in good to quantitative yields is reported. The thiol scope included alkyl and aryl thiols bearing electron-donating and electron-withdrawing groups. Short reaction time, high atom economy, inexpensive activator, free from workup and aryl halides, and gram-scale synthesis are the significant features
-
Gold(III)/Sodium Diphenylphosphinobenzene-3-sulfonate (TPPMS) Catalyzed Dehydrative N-Benzylation of Electron-Deficient Anilines in Water作者:Hidemasa Hikawa、Mika Matsumoto、Sayoko Tawara、Shoko Kikkawa、Isao AzumayaDOI:10.1055/s-0037-1611519日期:2019.7tolerates aerobic conditions. A Hammett study in the reaction of para-substituted benzhydryl alcohols shows negative σ values, indicating a build-up of cationic charge during the rate-determining sp3 C–O bond-cleavage step. The inverse kinetic solvent isotope effect (KSIE = 0.6) is consistent with a specific acid catalysis mechanism. This simple protocol can be performed under mild conditions in an atom-economic抽象的 已经开发出一种用于缺电子的苯胺在水中的脱水N-苄基化的策略。金(III)/二苯基膦基苯-3-磺酸钠(TPPMS)催化剂作为路易斯酸非常有效,可活化醇并耐受有氧条件。Hammett在对位取代的苯甲醇的反应中的研究显示出负σ值,表明在速率确定sp 3 C–O键断裂步骤期间阳离子电荷的积累。逆动力学溶剂同位素效应(KSIE = 0.6)与特定的酸催化机理一致。这种简单的方案可以在原子经济过程中的温和条件下进行,而无需碱或其他添加剂,从而提供了电子不足的N-苄基苯胺具有中等至优异的收率,并且水是唯一的副产物。 已经开发出一种用于缺电子的苯胺在水中的脱水N-苄基化的策略。金(III)/二苯基膦基苯-3-磺酸钠(TPPMS)催化剂作为路易斯酸非常有效,可活化醇并耐受有氧条件。Hammett在对位取代的苯甲醇的反应中的研究显示出负σ值,表明在速率确定sp 3 C–O键断裂步骤期间阳离子电荷的积累。逆动力学溶剂同位素效应(KSIE
-
Structure−Activity Relationship Studies of Novel 4-[2-[Bis(4-fluorophenyl)methoxy]ethyl]-1-(3-phenylpropyl)piperidine Analogs: Synthesis and Biological Evaluation at the Dopamine and Serotonin Transporter Sites作者:Aloke K. Dutta、Cen Xu、Maarten E. A. ReithDOI:10.1021/jm9506581日期:1996.1.1Several analogs of the potent dopamine (DA) transporter ligand 4-[2-[bis(4-fluorophenyl)-methoxy]ethyl]-1-(3-phenylpropyl)piperidine, 1b, were made and biologically evaluated for their binding at the DA and serotonin (5HT) transporters in rat striatal membranes. Different alkyl chain lengths and substitutions were introduced in these molecules to generate an optimum activity and selectivity for the制备了强效多巴胺(DA)转运蛋白配体4- [2- [双(4-氟苯基)-甲氧基]乙基] -1-(3-苯基丙基)哌啶的类似物1b,并对其在生物学上的结合进行了生物学评估。大鼠纹状体膜中的DA和血清素(5HT)转运蛋白。在这些分子中引入了不同的烷基链长度和取代基,以产生针对DA转运蛋白的最佳活性和选择性。通常,未取代的和氟取代的化合物对于DA转运蛋白是最活跃和最具选择性的。化合物4- [2-(二苯基甲氧基)乙基] -1-苄基哌啶9a显示出高效价,并且在该系列化合物中对DA转运蛋白的选择性最高(5HT / DA = 49)。发现其中一些新颖的类似物比原始GBR 12909分子在DA转运蛋白上的结合更具选择性,
-
Kinetics of the Solvolyses of Benzhydryl Derivatives: Basis for the Construction of a Comprehensive Nucleofugality Scale作者:Bernard Denegri、André Streiter、Sandra Jurić、Armin R. Ofial、Olga Kronja、Herbert MayrDOI:10.1002/chem.200500845日期:2006.2.8series of 21 benzhydrylium ions (diarylmethylium ions) are proposed as reference electrofuges for the development of a general nucleofugality scale, where nucleofugality refers to a combination of leaving group and solvent. A total of 167 solvolysis rate constants of benzhydrylium tosylates, bromides, chlorides, trifluoroacetates, 3,5-dinitrobenzoates, and 4-nitrobenzoates, two-thirds of which have been提出了一系列21个苯甲鎓离子(二芳基甲基鎓离子)作为参考电熔剂,用于开发一般的核易性规模,其中核易性是指离去基团和溶剂的组合。甲苯磺酸苄酯,溴化物,氯化物,三氟乙酸盐,3,5-二硝基苯甲酸酯和4-硝基苯甲酸酯的共167个溶剂分解速率常数,在这项工作中已确定其中三分之二,根据相关方程log k(25摄氏度)= sf(Nf + Ef),其中sf和Nf是核反应堆特定的参数,Ef是电堆特定的参数。尽管以这种方式表征的核熔剂和电熔剂覆盖了12个数量级以上,但是只有一组参数,即sf,Nf和Ef,足以计算出25°C时的溶剂分解速率常数,精度为+/- 16%。到目前为止,由于所有核因子的sf大约为1,即离开基团/溶剂的组合,因此对核官能度的定性讨论可以基于Nf。
-
Synthesis and insulin-sensitizing activity of (<i>S</i>)-2-ethoxy-3-phenylpropanoic acid derivatives作者:Xiao-hua Cai、Bing XieDOI:10.1139/v06-145日期:2006.9.1
A series of (S)-2-ethoxy-3-phenylpropanoic acid derivatives were synthesized and their insulin-sensitizing activities were evaluated in 3T3-L1 cells. Compounds 1b (EC30 = 9.43 × 10–3 µmol/L), 1d (EC30 = 7.45 × 10–3 µmol/L), 1e (EC30 = 6.22 × 10–3 µmol/L), and 1f (EC30 = 7.76 × 10–3 µmol/L) exhibited more potent insulin-sensitizing activity than rosiglitazone (EC30 = 2.06 × 10–2 µmol/L).Key words: (S)-2-ethoxy-3-phenylpropanoic acid derivatives, type 2 diabetes, insulin-sensitizing agents.
表征谱图
-
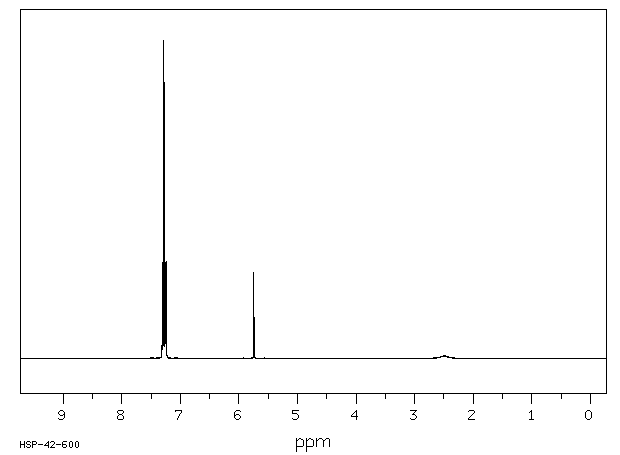
氢谱1HNMR
-
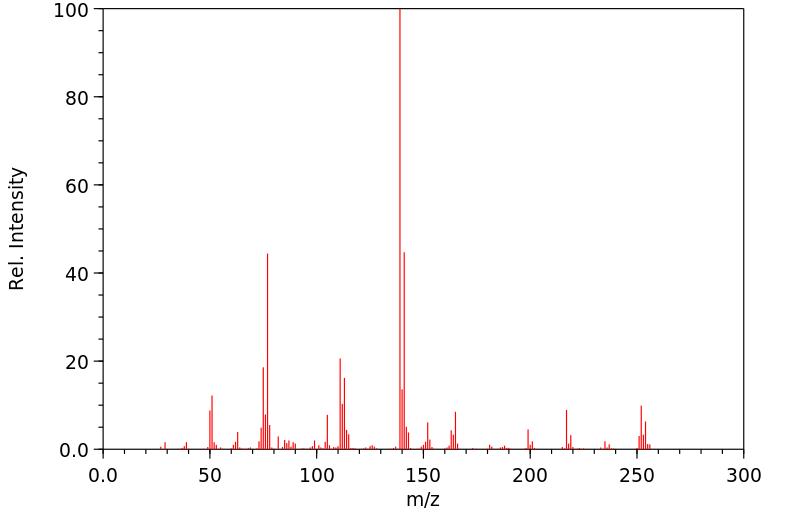
质谱MS
-
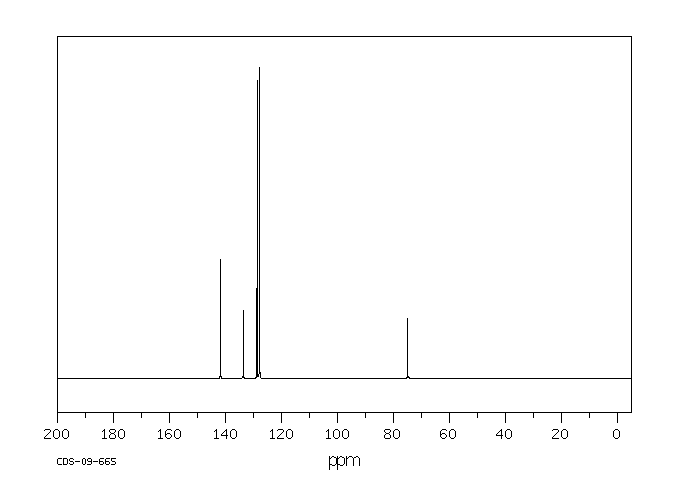
碳谱13CNMR
-
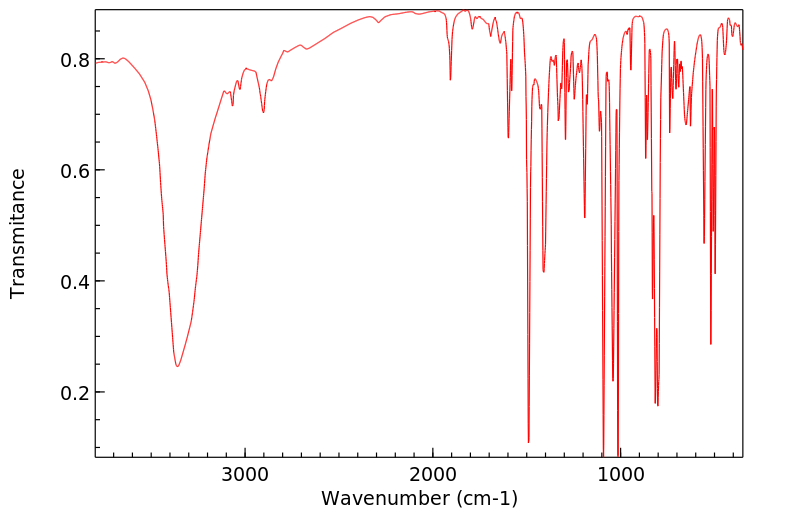
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫