2-methoxy-10H-acridine-9-thione | 21516-39-2
中文名称
——
中文别名
——
英文名称
2-methoxy-10H-acridine-9-thione
英文别名
2-Methoxy-10H-acridin-9-thion;2-methoxy-acridane-9-thione;2-Methoxy-thio-acridon-(9);Lbdmftsefyrpqz-uhfffaoysa-;2-methoxy-10H-acridine-9-thione
CAS
21516-39-2
化学式
C14H11NOS
mdl
——
分子量
241.313
InChiKey
LBDMFTSEFYRPQZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:232-233.5 °C
-
沸点:420.7±55.0 °C(Predicted)
-
密度:1.32±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:53.4
-
氢给体数:1
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 9-氯-2-甲氧基吖啶 9-chloro-2-methoxyacridine 16492-13-0 C14H10ClNO 243.692 —— 2-methoxy-9(10H)-acridinone 49742-72-5 C14H11NO2 225.247
反应信息
-
作为反应物:描述:参考文献:名称:Structure–activity relationship study of acridine analogs as haspin and DYRK2 kinase inhibitors摘要:Haspin is a serine/threonine kinase required for completion of normal mitosis that is highly expressed during cell proliferation, including in a number of neoplasms. Consequently, it has emerged as a potential therapeutic target in oncology. A high throughput screen of approximately 140,000 compounds identified an acridine analog as a potent haspin kinase inhibitor. Profiling against a panel of 270 kinases revealed that the compound also exhibited potent inhibitory activity for DYRK2, another serine/threonine kinase. An optimization study of the acridine series revealed that the structure-activity relationship (SAR) of the acridine series for haspin and DYRK2 inhibition had many similarities. However, several structural differences were noted that allowed generation of a potent haspin kinase inhibitor (33, IC(50) <60 nM) with 180fold selectivity over DYRK2. In addition, a moderately potent DYRK2 inhibitor (41, IC(50) <400 nM) with a 5.4-fold selectivity over haspin was also identified. (C) 2010 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2010.04.150
-
作为产物:描述:参考文献:名称:Structure–activity relationship study of acridine analogs as haspin and DYRK2 kinase inhibitors摘要:Haspin is a serine/threonine kinase required for completion of normal mitosis that is highly expressed during cell proliferation, including in a number of neoplasms. Consequently, it has emerged as a potential therapeutic target in oncology. A high throughput screen of approximately 140,000 compounds identified an acridine analog as a potent haspin kinase inhibitor. Profiling against a panel of 270 kinases revealed that the compound also exhibited potent inhibitory activity for DYRK2, another serine/threonine kinase. An optimization study of the acridine series revealed that the structure-activity relationship (SAR) of the acridine series for haspin and DYRK2 inhibition had many similarities. However, several structural differences were noted that allowed generation of a potent haspin kinase inhibitor (33, IC(50) <60 nM) with 180fold selectivity over DYRK2. In addition, a moderately potent DYRK2 inhibitor (41, IC(50) <400 nM) with a 5.4-fold selectivity over haspin was also identified. (C) 2010 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2010.04.150
文献信息
-
Poulallion, Pierre; Galy, Jean-Pierre; Vincent, Emile-Jean, Journal of Heterocyclic Chemistry, 1986, vol. 23, p. 1141 - 1149作者:Poulallion, Pierre、Galy, Jean-Pierre、Vincent, Emile-Jean、Galy, Anne-Marie、Barbe, Jacques、Atassi, GhanemDOI:——日期:——
-
Synthesis and biological properties of derivatives of 4-heterylmercaptoquinazoline作者:R. S. Sinyak、I. A. Mazur、V. R. Smets、A. A. Martynovskii、P. N. SteblyukDOI:10.1007/bf00766901日期:1986.2
-
Tschernzow, Zhurnal Obshchei Khimii, 1944, vol. 14, p. 186,192作者:TschernzowDOI:——日期:——
-
Das-Gupta, Journal of the Indian Chemical Society, 1940, vol. 17, p. 246作者:Das-GuptaDOI:——日期:——
-
MARTYNOVSKIJ, A. A.;KLYUEV, N. A.;KURAPOV, P. B.;MELNIK, I. V.;PANASENKO,+, IZV. TIMIRYAZEV. S.-X. AKAD.,(1989) N, S. 157-165作者:MARTYNOVSKIJ, A. A.、KLYUEV, N. A.、KURAPOV, P. B.、MELNIK, I. V.、PANASENKO,+DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
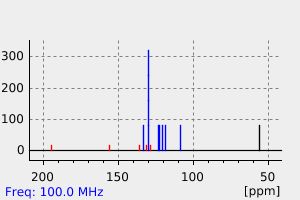
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43