1-(4-氯苯基)咪唑 | 51581-54-5
中文名称
1-(4-氯苯基)咪唑
中文别名
——
英文名称
1-(4-chlorophenyl)imidazole
英文别名
1-(4-chlorophenyl)-1H-imidazole;N-(4-chlorophenyl)imidazole
CAS
51581-54-5
化学式
C9H7ClN2
mdl
MFCD00041208
分子量
178.621
InChiKey
BARLRKAYTDVUIS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:85-87°C
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
海关编码:2933290090
-
安全说明:S26,S36/37/39
-
WGK Germany:3
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(1H-咪唑-1-基)苯胺 4-(N-imidazolyl)aniline 2221-00-3 C9H9N3 159.191 4'-(咪唑-1-基)苯腈 4-(1H-imidazol-1-yl)benzonitrile 25372-03-6 C10H7N3 169.186 1-(4-联苯基)-1H-咪唑 1-biphenyl-4-yl-1H-imidazole 108085-60-5 C15H12N2 220.274
反应信息
-
作为反应物:描述:1-(4-氯苯基)咪唑 在 ammonium hydroxide 、 potassium phosphate 、 copper(l) iodide 、 N1,N2-bis(5-methyl-[1,1'-biphenyl]-2-yl)oxalamide 作用下, 以 水 、 二甲基亚砜 为溶剂, 反应 24.0h, 以88%的产率得到4-(1H-咪唑-1-基)苯胺参考文献:名称:通过CuI /羟基二酰胺催化的芳基氯化物和氨气耦合组装伯(杂)芳胺摘要:已经开发了一种通用的和实用的催化体系,用于用含水或气态氨将芳基氯化物进行芳基胺化,其中CuI为催化剂,双芳基草酸二酰胺为配体。反应在105–120°C下进行,以高收率提供具有各种官能团的各种伯(杂)芳基胺。DOI:10.1021/acs.orglett.5b03230
-
作为产物:描述:参考文献:名称:对位取代对TAAILs熔点的电子影响摘要:由于大量新应用,在过去十年中,对“特定任务”离子液体的兴趣大大增加。但是,不幸的是,基于咪唑鎓的离子液体(迄今为止最常用的阳离子)在改变其性能方面有严重的局限性。新一代离子液体,称为可调式芳基烷基离子液体(TAAIL),用芳基环取代了咪唑鎓环上的两个烷基链之一,从而实现了很大程度的功能化。感性,内消旋,和位阻效应以及可能还π π和π π +交互作用提供了广泛的可能性来调整此类新的IL。通过研究熔点的变化,我们研究了在芳环对位的吸电子和给电子取代基的影响(NO 2,Cl,Br,EtO(CO),H,Me,OEt,OMe)相应的溴化物和双(三氟甲烷磺酰)亚胺,(N(TF)2 - ),其盐。此外,我们计算了(B3LYP / 6-311 ++ G(d,p))取代的1-芳基-3-丙丙基咪唑鎓阳离子的不同电荷分布,以了解实验观察到的效果。结果表明,给电子和吸电子基团的存在导致阳离子中强烈的极化效应。DOI:10.1002/asia.201000744
文献信息
-
Room-Temperature Copper-Catalyzed Carbon-Nitrogen Coupling of Aryl Iodides and Bromides Promoted by Organic Ionic Bases作者:Chu-Ting Yang、Yao Fu、Yao-Bing Huang、Jun Yi、Qing-Xiang Guo、Lei LiuDOI:10.1002/anie.200903158日期:2009.9.21solubility alone does not explain the performance of organic ionic bases in the room‐temperature coupling of aryl iodides and even bromides with aliphatic and aromatic amines and N‐heterocycles (NuH; see scheme). Conductivity measurements show that these organic ionic bases, which contain tetraalkylammonium or ‐phosphonium cations, are readily ionized in organic solvents.
-
Air-stable palladium(0) phosphine sulfide catalysts for Ullmann-type C–N and C–O coupling reactions作者:Arpi Majumder、Ragini Gupta、Mrinmay Mandal、Madhu Babu、Debashis ChakrabortyDOI:10.1016/j.jorganchem.2014.11.018日期:2015.4N-arylation and O-arylation of aryl halides by Ullmann-type cross coupling reaction under mild reaction conditions in a short reaction time. Two phosphine sulphide ligands and their corresponding Pd(0) complexes namely [Pd(p2S2)(dba)] and [Pd(pp3S4)(dba)], were synthesized, where p2S2 is 1,2-bis(diphenylphosphino)ethane disulfide, pp3S4 is tris[2-(diphenylphosphino)ethyl]phosphine tetrasulfide and dba本文描述了在温和的反应条件下,在较短的反应时间内,钯(0)催化Ullmann型交叉偶联反应进行芳基卤化物的N-芳基化和O-芳基化的有效方法。合成了两个硫化膦配体及其相应的Pd(0)络合物,即[Pd(p 2 S 2)(dba)]和[Pd(pp 3 S 4)(dba)],其中p 2 S 2为1, 2-双(二苯基膦基)乙烷二硫化物,pp 3 S 4是三[2-(二苯基膦基)乙基]膦四硫化物,dba是二亚苄基丙酮。通过改变温度,溶剂,碱和催化剂的负载量,确定了使用碘代苯和苯并咪唑进行芳基化反应的最佳反应条件。使用碘代苯/溴苯和具有不同空间和电子性质的各种取代的芳基胺/苯酚/醇进行交叉偶联反应,从而以良好或优异的收率得到所需的N-芳基胺/二芳基醚/烷基芳基醚( 70–94%)。
-
Open-air N-arylation of N–H heterocycles with arylboronic acids catalyzed by copper(II) Schiff base complexes作者:S. M. Islam、Ram Chandra Dey、Anupam Singha Roy、Sumantra Paul、Sanchita MondalDOI:10.1007/s11243-014-9881-2日期:2014.11Two copper Schiff base complexes, in both homogeneous and heterogeneous forms, were prepared and characterized by using elemental analysis, FTIR, UV–Vis spectroscopy and scanning electron microscopy. The catalytic performances of these complexes were studied in the N-arylation of N–H heterocycles with arylboronic acids in methanol without any added base at 40 °C under open air. The effects of various parameters such as solvent and temperature on the reaction system were studied. The reaction is applicable to a wide variety of N–H heterocycles and arylboronic acids. The heterogeneous catalyst was recovered by simple filtration, and reusability experiments showed that this catalyst can be used five times without much loss in the catalytic activity.
-
CuI/Oxalamide Catalyzed Couplings of (Hetero)aryl Chlorides and Phenols for Diaryl Ether Formation作者:Mengyang Fan、Wei Zhou、Yongwen Jiang、Dawei MaDOI:10.1002/anie.201601035日期:2016.5.17Couplings between (hetero)aryl chlorides and phenols can be effectively promoted by CuI in combination with an N‐aryl‐N′‐alkyl‐substituted oxalamide ligand to proceed smoothly at 120 °C. For this process, N‐aryl‐N′‐alkyl‐substituted oxalamides are more effective ligands than bis(N‐aryl)‐substituted oxalamides. A wide range of electron‐rich and electron‐poor aryl and heteroaryl chlorides gave the corresponding
-
Cross-Coupling Reactions of Aryl Halides with Amines, Phenols, and Thiols Catalyzed by an N,N′-Dioxide-Copper(I) Catalytic System作者:Haitao Yang、Chao Xi、Zhiwei Miao、Ruyu ChenDOI:10.1002/ejoc.201100274日期:2011.6reactions of various N, O, and S nucleophilic reagents with aryl halides have been successfully carried out under mild conditions by using a novel chiral N,N′-dioxide–copper(I) catalytic system as the catalyst. This versatile and efficient catalyst system has been demonstrated to facilitate the cross-coupling reactions of aryl halides with amines,phenols, and thiols to afford the corresponding desired
表征谱图
-
氢谱1HNMR
-
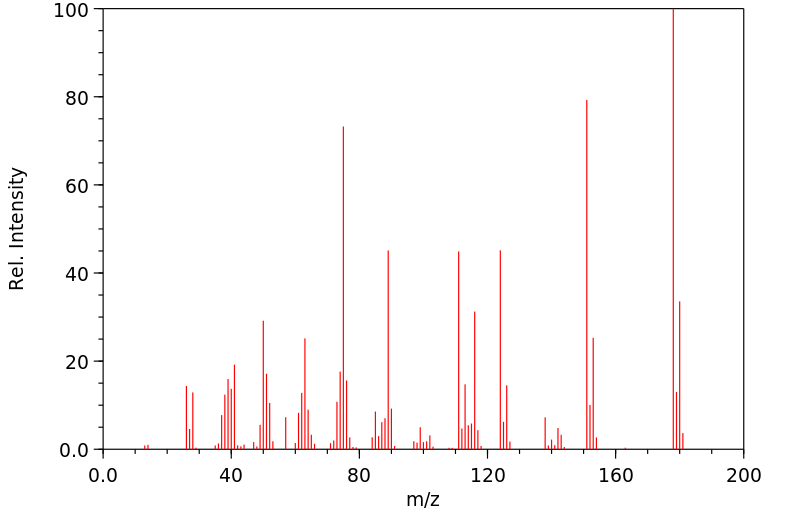
质谱MS
-
碳谱13CNMR
-
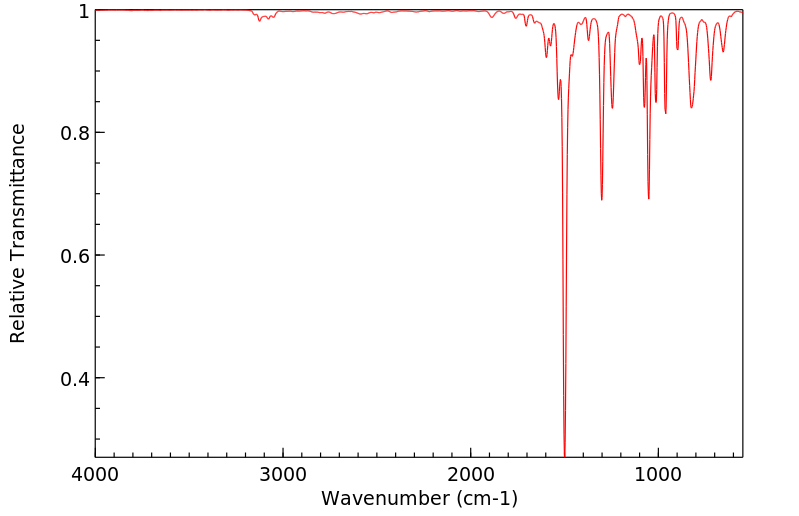
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)