氯乙酸苄酯 | 140-18-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:90 °C/1 mmHg (lit.)
-
密度:1.215 g/mL at 25 °C (lit.)
-
闪点:>230 °F
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2915400090
-
RTECS号:AF8750000
-
储存条件:本品应密封保存。
SDS
: 氯乙酸苄酯
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H9ClO2
分子式
: 184.62 g/mol
分子量
组分 浓度或浓度范围
Phenylmethyl chloroacetate
-
CAS 号 140-18-1
EC-编号 205-400-0
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氯化氢气体
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 将人员撤离到安全区域。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用惰性吸附材料吸收并当作危险废品处理。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
90 °C 在 1 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
1.215 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: AF8750000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙醇酸苯甲酯 benzyl glycolate 30379-58-9 C9H10O3 166.177 碘乙酸苄基酯 benzyl iodoacetate 81867-37-0 C9H9IO2 276.074 2-溴乙酸苄酯 Benzyl bromoacetate 5437-45-6 C9H9BrO2 229.073 —— benzyl diazoacetate 52267-51-3 C9H8N2O2 176.175 —— monochloroacetaldehyde dibenzyl acetal 37003-25-1 C16H17ClO2 276.763 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 乙酸苄酯 Benzyl acetate 140-11-4 C9H10O2 150.177 —— benzyl fluoroacetate 458-77-5 C9H9FO2 168.168 碘乙酸苄基酯 benzyl iodoacetate 81867-37-0 C9H9IO2 276.074 —— benzyl 2-chloro-3-oxopropanoate 1313409-83-4 C10H9ClO3 212.633 —— benzyl diazoacetate 52267-51-3 C9H8N2O2 176.175 苄基苄基氧基乙酸酯 benzyl benzyloxyacetate 30379-54-5 C16H16O3 256.301 乙酸,叠氮-,苯基甲基酯 benzyl 2-azidoacetate 173601-46-2 C9H9N3O2 191.189 —— 2-(dimethylamino)acetic acid benzyl ester 30379-57-8 C11H15NO2 193.246 —— (E)-benzyl pent-3-enoate —— C12H14O2 190.242 2-(苯基甲氧羰基甲基硫基)乙酸苄基酯 sulfanediyldi-acetic acid dibenzyl ester 7497-95-2 C18H18O4S 330.405 —— benzyl acetoxyacetate 30379-73-8 C11H12O4 208.214 —— benzyl isopropylglycinate —— C12H17NO2 207.272 —— benzyl 3-methylbut-3-enoate 37526-86-6 C12H14O2 190.242 —— dibenzyl diglycolyl diglycolate 1135131-59-7 C22H22O9 430.411 —— benzyl 2-(diethylamino)acetate 53342-20-4 C13H19NO2 221.299 —— (E)-cinnamic acid benzyl ester 103-41-3 C16H14O2 238.286 —— 2-Benzyl-1-methyl-1,1-dithiooxalat 69443-02-3 C10H10O2S2 226.32 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:无水氯化铁和羰基rh化合物催化的苄基酯的脱保护摘要:无水氯化铁和[Re(CO)4 Br] 2被证明是用于苄基酯催化脱保护的有用试剂。合适的保护基是对-MeC 6 H 4 CH 2,其用于脱苄基的工作温度为50°C。DOI:10.1016/s0040-4039(01)02152-9
-
作为产物:参考文献:名称:FeCl2介导的菲咯啉配体加速碘代烷的亲核氯化摘要:Alkyl halides have been considered not only important building blocks but also useful intermediates in synthetic methods of nucleophilic substitution, metal-halogen exchange, and cross-coupling reactions. As the practical synthesis of various alkyl halides, the halogen exchange is one of the fundamental and efficient reactions, which is mainly used for preparing alkyl bromides or iodides. Finkelstein-type reactions(1) have been well studied for the conversion of alkyl chlorides to the corresponding bromides or iodides and alkyl bromides to iodides, which reaction is an equilibrium shifted by taking advantage of the different solubility of the resulting sodium salt in acetone. However, the reverse processes, the replacement of iodide by bromide or chloride, or of bromide by chloride, were not simple.DOI:10.1002/bkcs.11453
文献信息
-
The Hydrolysis of Esters Related to <i>O</i>-Hippuryl-2-hydroxybutanoic Acid by Carboxypeptidase A作者:John W. Bunting、Joe MurphyDOI:10.1139/v74-385日期:1974.7.15
The hydrolysis of each of the following esters by bovine carboxypeptidase A has been studied at pH 7.5, 25°, ionic strength 0.5: O-hippuryl-, O-phenaceturyl-, O-aceturyl-, O-(N-methylhippuryl)-, and O-(N-hippurylglycyl)-2-hydroxybutanoic acids, and 2-(3-benzoylpropanoxy)-, 2-benzoxyacetoxy-, and 2-(4-phenylbutanoxy)butanoic acids. Substrate inhibition occurs with only the hippuric and phenaceturic acid esters and in the six other cases simple Michaelis–Menten kinetics are observed. The relatively minor variations in the structures of the acid moieties of these esters lead to quite large variations in Km, although kcat seems to be relatively independent of the nature of the acid moiety. Binding modes of substrate molecules at both the catalytic and inhibitory sites are discussed in the light of these observations.
-
Synthesis of some acyclic quaternary ammonium compounds. Alkylation of secondary and tertiary amines in a two-phase system作者:A. V. Kharlamov、O. I. Artyushin、N. A. BondarenkoDOI:10.1007/s11172-014-0761-x日期:2014.11and asymmetrical quaternary ammonium chlorides of the general formula R1R2R3N+AR4Cl– (R1 = Me, Bu; R2 = n-C12H25, PhCH2, CnH2n+1(OCH2CH2)m, n = 9 and 12, m = 1 and 2; R3 = n-C12H25, PhCH2, HOCH2CH2,–OOCCH2; R4 = n-C12H25, PhCH2; A = (CH2CH2O)1,2, CH2C(O)O) was synthesized by the alkylation of tertiary amines in a two-phase system containing water. A convenient method for the synthesis of the initial通式R1R2R3N+AR4Cl–(R1 = Me, Bu; R2 = n-C12H25, PhCH2, CnH2n+1(OCH2CH2)m, n = 9 and 12, m = 1)的一系列无环对称和不对称季铵氯化物和 2; R3 = n- , PhCH2, HOCH2CH2,–OOCCH2; R4 = n- , PhCH2; A = (CH2CH2O)1,2, CH2C(O)O) 是通过叔胺的烷基化合成的相系统含水。一种合成通式 MeNR1R2 的初始对称和不对称叔胺的简便方法(R1 = Me, Bu;R2 = n- , PhCH2, CnH2n+1(OCH2CH2)m, n = 9 和 12, m = 1 和 2) 在有机相-水相非均相系统中被开发出来,它允许使用碱和胺的水溶液。
-
Preparation of Various Carboxylic Acid Esters from Bulky Alcohols and Carboxylic Acids by a New Type Oxidation-reduction Condensation Using 2,6-Dimethyl-1,4-benzoquinone作者:Teruaki Mukaiyama、Wataru Kikuchi、Taichi ShintouDOI:10.1246/cl.2003.300日期:2003.3A new-type oxidation-reduction condensation by using 2,6-dimethyl-1,4-benzoquinone (DMBQ), carboxylic acids and in situ formed alkoxydiphenylphosphines (1) including the bulky alkoxy group-substituted ones proceeded smoothly to afford the corresponding carboxylic acid esters in good to high yields. Alkoxydiphenylphosphines were formed in situ by treating either N,N-dimethylaminodiphenylphosphine (Ph2PNMe2)
-
REDUCTION OF α-HALOCARBONYL COMPOUNDS WITH SODIUM HYDROGEN TELLURIDE作者:Atsuhiro Osuka、Hitomi SuzukiDOI:10.1246/cl.1983.119日期:1983.1.5Sodium hydrogen telluride is an efficient reagent for dehalogenation of α-halocarbonyl compounds.氢碲化钠是一种高效的α-卤代羰基化合物脱卤试剂。
-
Efficient Method for the Preparation of Carboxylic Acid Alkyl Esters or Alkyl Phenyl Ethers by a New-Type of Oxidation–Reduction Condensation Using 2,6-Dimethyl-1,4-benzoquinone and Alkoxydiphenylphosphines作者:Taichi Shintou、Wataru Kikuchi、Teruaki MukaiyamaDOI:10.1246/bcsj.76.1645日期:2003.8A new-type of oxidation–reduction condensation proceeded smoothly to afford carboxylic acid alkyl esters or alkyl phenyl ethers in good to high yields by combined use of alkoxydiphenylphosphines (1...一种新型氧化还原缩合反应顺利进行,通过结合使用烷氧基二苯基膦(1...
表征谱图
-
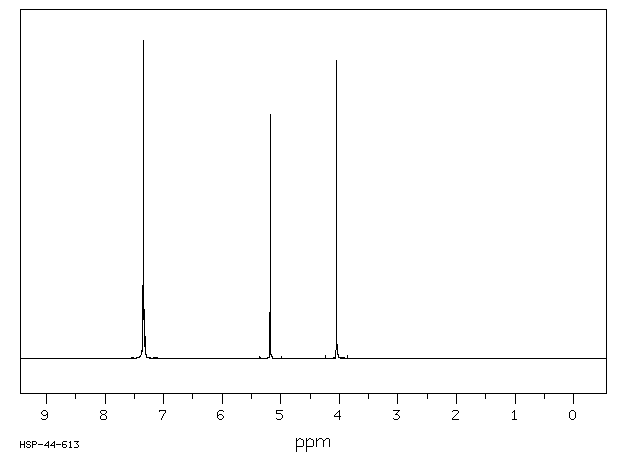
氢谱1HNMR
-
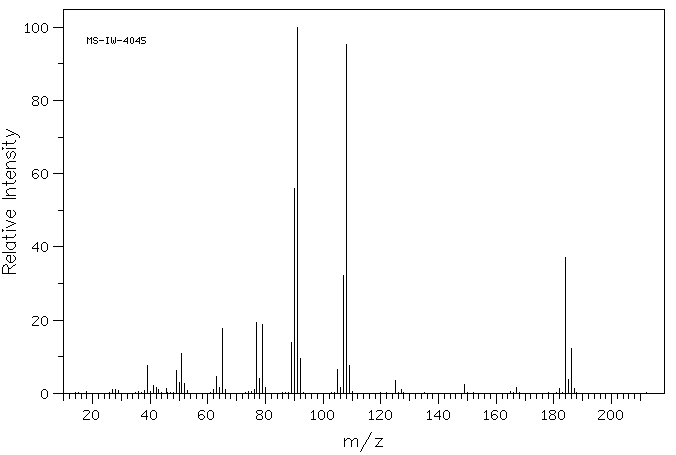
质谱MS
-
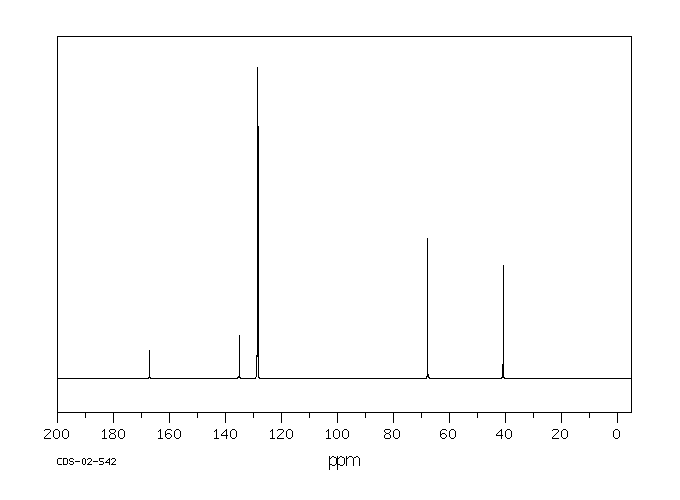
碳谱13CNMR
-
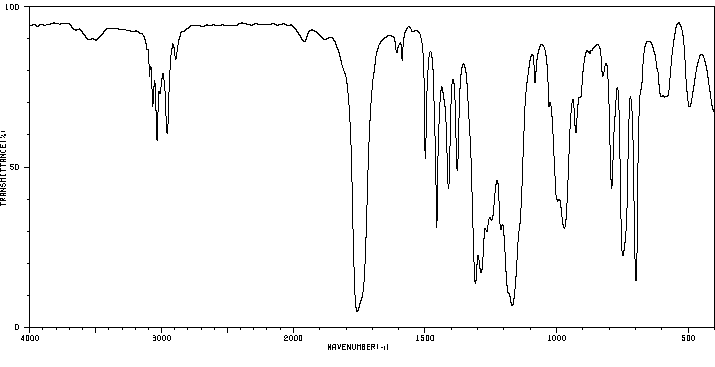
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息