氯乙酸苯酯 | 620-73-5
中文名称
氯乙酸苯酯
中文别名
氯醋酸苯酯
英文名称
phenyl chloroacetate
英文别名
phenyl 2-chloroacetate;chloroacetic acid phenyl ester
CAS
620-73-5
化学式
C8H7ClO2
mdl
MFCD00018921
分子量
170.595
InChiKey
AGUWUIVKDXDKBT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:44.5°C
-
沸点:241.44°C (rough estimate)
-
密度:1.2202
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915400090
SDS
制备方法与用途
化学性质
性状:无色透明液体
用途:作为中间体使用
上下游信息
反应信息
-
作为反应物:描述:氯乙酸苯酯 在 盐酸 、 aluminum (III) chloride 、 sodium acetate 作用下, 以 甲醇 、 水 、 异丙醇 为溶剂, 反应 1.0h, 生成 2-hydroxy-5-{[(2Z)-3-oxobenzofuran-2-ylidene]methyl}benzoic acid参考文献:名称:黄酮启发了发现亚苄基苯并呋喃-3(2H)-ones(aurones)作为人类蛋白激酶CK2的有效抑制剂的发现。摘要:在这项工作中,我们描述了2-亚苄基苯并呋喃-3-酮(aurones)的设计,合成和SAR研究,该化合物是CK2的新型强效抑制剂。已经合成了一系列的金酮。这些化合物在结构上与合成的黄酮有关,并显示出对CK2的纳摩尔活性。生化测试表明,有20种新合成的化合物抑制CK2,IC 50值在纳摩尔范围内。进行了进一步的基于属性的金质优化,产生了一系列亲脂效率增强的CK2抑制剂。最有效的化合物12m(BFO13)的CLipE = 4.94(CLogP = 3.5; IC 50 = 3.6 nM)与最知名的CK2抑制剂相当。DOI:10.1016/j.bioorg.2020.104062
-
作为产物:参考文献:名称:The Preparation and Alcoholysis of Phenyl Iminoester Hydrochlorides摘要:DOI:10.1021/ja01220a507
文献信息
-
Design, synthesis and biological evaluation of uncharged catechol derivatives as selective inhibitors of PTP1B作者:Xiang-Qian Li、Qi Xu、Jiao Luo、Li-Jun Wang、Bo Jiang、Ren-Shuai Zhang、Da-Yong ShiDOI:10.1016/j.ejmech.2017.05.007日期:2017.8obesity. However, the development of charged PTP1B inhibitors was restricted due to their low cell permeability and poor bioavailability. Based on active natural products, two series of uncharged catechol derivatives were identified as PTP1B inhibitors by targeting a secondary aryl phosphate-binding site as well as the catalytic site. The most potent inhibitor 22 showed an IC50 of 0.487 μM against PTP1B and蛋白质酪氨酸磷酸酶1B(PTP1B)是有效治疗T2DM和肥胖症的有希望且经过验证的治疗靶标。但是,带电的PTP1B抑制剂由于其低的细胞渗透性和较差的生物利用度而受到限制。基于活性天然产物,通过靶向磷酸二芳基酯结合位点和催化位点,鉴定了两个系列的不带电荷的邻苯二酚衍生物为PTP1B抑制剂。最有效的抑制剂22对PTP1B的IC 50为0.487μM,相对于TCPTP具有很强的选择性(27倍)。还进行了动力学研究,发现22种可作为竞争性PTP1B抑制剂。22例C2C12肌管的治疗显着增加了IRβ,Akt和IRS1的磷酸化水平。其作用谱与胰岛素产生的相似,表明其作为一种新的非胰岛素依赖性候选药物的潜力。
-
An Intramolecular Wittig Approach toward Heteroarenes: Synthesis of Pyrazoles, Isoxazoles, and Chromenone-oximes作者:Pankaj V. Khairnar、Tsai-Hui Lung、Yi-Jung Lin、Chi-Yi Wu、Srinivasa Rao Koppolu、Athukuri Edukondalu、Praneeth Karanam、Wenwei LinDOI:10.1021/acs.orglett.9b01395日期:2019.6.7α-Halohydrazones/ketoximes are transformed into trisubstituted pyrazoles/disubstituted isoxazoles by treatment with phosphine, acyl chloride, and a base. Mechanistic investigations revealed the in situ formation of azo/nitroso olefin intermediates which underwent a tandem phospha-Michael/N- or O-acylation/intramolecular Wittig reaction to afford the heteroarenes in moderate to good yields. Further
-
One-pot synthesis of indolizine via 1,3-dipolar cycloaddition using a sub-equivalent amount of K<sub>2</sub>Cr<sub>2</sub>O<sub>7</sub> as an efficient oxidant under base free conditions
-
Process for preparing phenyl esters of substituted acids
-
Electron-Withdrawing Substituents Decrease the Electrophilicity of the Carbonyl Carbon. An Investigation with the Aid of <sup>13</sup>C NMR Chemical Shifts, ν(CO) Frequency Values, Charge Densities, and Isodesmic Reactions To Interprete Substituent Effects on Reactivity作者:Helmi Neuvonen、Kari Neuvonen、Andreas Koch、Erich Kleinpeter、Paavo PasanenDOI:10.1021/jo020121c日期:2002.10.1electron-withdrawing substituents destabilize the carbonyl derivatives investigated. So, a significant ground-state destabilization of carboxylic acid esters, and carbonyl compounds in general, due to the decreased resonance stabilization, is proposed as a novel concept to explain both the increase in their reactivity and the changes in the chemical shifts and carbonyl frequencies induced by electron-withdrawing
表征谱图
-
氢谱1HNMR
-
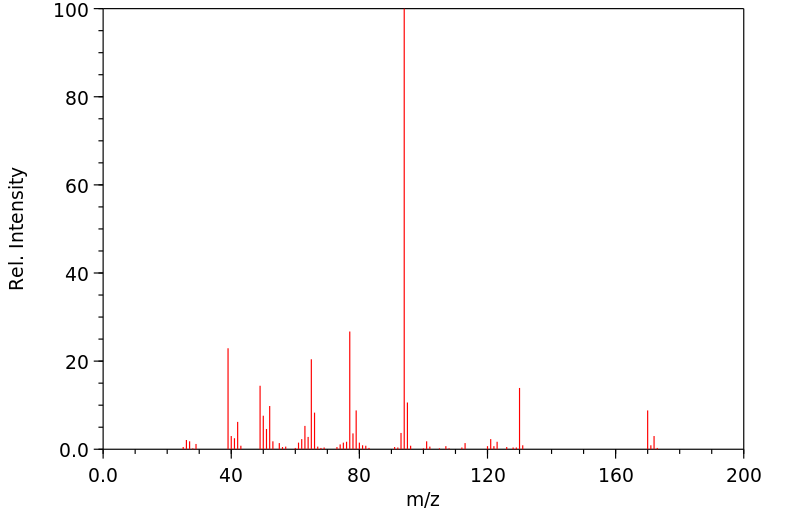
质谱MS
-
碳谱13CNMR
-
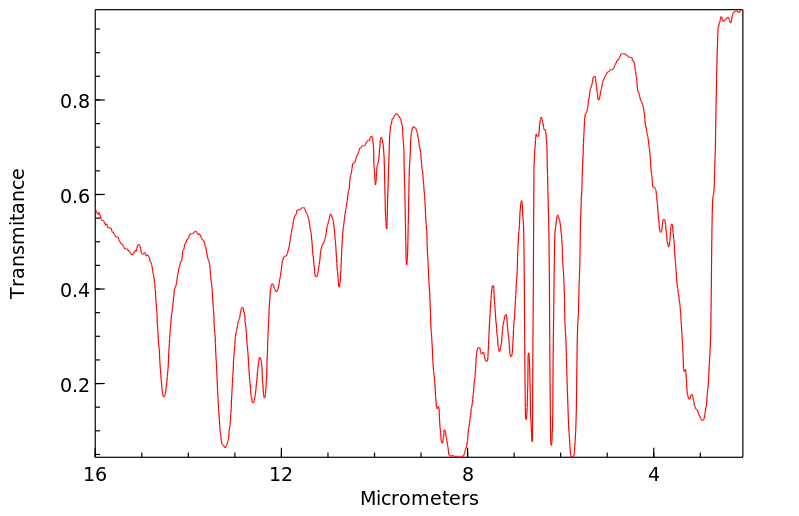
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)