5,7-二甲氧基香豆素 | 487-06-9
中文名称
5,7-二甲氧基香豆素
中文别名
5,7-二甲氧香豆素;柠美内酯;白檬素;5,7-二甲氧基香豆素(Citropten)
英文名称
Limettin
英文别名
5,7-dimethoxycoumarin;citropten;5,7-dimethoxy-2H-chromen-2-one;5,7-dimethoxy-2H-1-benzopyran-2-one;5,7-dimethoxychromen-2-one
CAS
487-06-9
化学式
C11H10O4
mdl
MFCD00006870
分子量
206.198
InChiKey
NXJCRELRQHZBQA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:146-149 °C (lit.)
-
沸点:305.04°C (rough estimate)
-
密度:1.2607 (rough estimate)
-
溶解度:可溶于乙腈(少许)、氯仿(少许)
-
LogP:1.891 (est)
-
物理描述:Solid
-
碰撞截面:138.3 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
保留指数:1916;1916
-
稳定性/保质期:
按照规定使用和储存,不会发生分解,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.181
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S36/37
-
危险类别码:R43
-
WGK Germany:3
-
RTECS号:GN6530000
-
危险标志:GHS07
-
危险性描述:H317
-
危险性防范说明:P280
-
储存条件:温度:2-8°C 储存方式:密封保存,放置于通风、干燥处。目前暂无其他存储建议。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 5,7-Dimethoxycoumarin
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 487-06-9
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Skin sensitisation (Category 1), H317
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xi Irritant R43
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Warning
Hazard statement(s)
H317 May cause an allergic skin reaction.
Precautionary statement(s)
P280 Wear protective gloves.
Supplemental Hazard none
Statements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Synonyms : Citropten
Limettin
Formula : C11H10O4
Molecular Weight : 206,19 g/mol
CAS-No. : 487-06-9
EC-No. : 207-646-4
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
5,7-Dimethoxy-2-benzopyrone
CAS-No. 487-06-9 Skin Sens. 1; H317 <= 100 %
EC-No. 207-646-4
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
5,7-Dimethoxy-2-benzopyrone
CAS-No. 487-06-9 Xi, R43 <= 100 %
EC-No. 207-646-4
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: crystalline
Colour: white
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 146 - 149 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
Germ cell mutagenicity
Human
lymphocyte
Sister chromatid exchange
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
photosensitivity, To the best of our knowledge, the chemical, physical, and toxicological properties have
not been thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-羟基-7-甲氧基香豆素 5,7-dihydroxycoumarin 7-methyl ether 23053-61-4 C10H8O4 192.171 5,7-二羟基香豆素 5,7-dihydroxy-2H-chromen-2-one 2732-18-5 C9H6O4 178.144 5-香叶氧基-7-甲氧基香豆素 5-geranyloxy-7-methoxycoumarin 7380-39-4 C20H24O4 328.408 —— 5,7-dimethoxy-2-oxo-2H-chromene-3-carboxylic acid 81017-27-8 C12H10O6 250.208 —— ethyl 5,7-dimethoxy-2-oxo-2H-chromene-3-carboxylate 82235-61-8 C14H14O6 278.262 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 白蜡树精 fraxinol 486-28-2 C11H10O5 222.197 —— 8-hydroxy-5,7-dimethoxycoumarin (leptodactylone) 61899-44-3 C11H10O5 222.197 —— acetyl fraxinol 28752-90-1 C13H12O6 264.235 —— 8-formyllimettin 88140-30-1 C12H10O5 234.208 —— murrayacarpin B —— C12H12O5 236.224 —— 5,7-dimethoxy-4-trifluoromethylsulfonyloxychromen-2-one 185418-17-1 C12H9F3O7S 354.26 月橘香豆精 coumurrayin 17245-25-9 C16H18O4 274.317 —— toddalenone 88142-59-0 C15H14O5 274.273 —— gleinadiene —— C16H16O4 272.301
反应信息
-
作为反应物:描述:参考文献:名称:Bhavsar; Desai, Indian Journal of Pharmacy, 1951, vol. 13, p. 200,203摘要:DOI:
-
作为产物:描述:2-formyl-3,5-dimethoxyphenyl 2-chloroacetate 以 N,N-二甲基甲酰胺 为溶剂, 以35%的产率得到5,7-二甲氧基香豆素参考文献:名称:在银阴极还原取代的 2-氯乙酸苯酯:香豆素的电合成摘要:To explore the electrosynthesis of coumarins, cyclic voltammetry and controlled-potential (bulk) electrolysis have been employed to investigate the reduction of the carbon-chlorine bond of five substituted phenyl 2-chloroacetates at silver cathodes in dimethylformamide (DMF) containing 0.10 M tetra-n-butylammonium tetrafluoroborate (TBABF(4)) as supporting electrolyte; the five substrates are 2-formylphenyl 2-chloroacetate (1a), 2-acetylphenyl 2-chloroacetate (2a), methyl 2-(2-chloroacetoxy)benzoate (3a), 2-formyl-5-methoxyphenyl 2-chloroacetate (4a), and 2-formyl-3,5-dimethoxyphenyl 2-chloroacetate (5a). We have examined (a) the effects of substituents on the benzene ring of the substrate as well as the nature of the aryl carbonyl moiety on the formation of the coumarin product and (b) the effect of solvent-namely, DMF, acetonitrile (CH3CN), benzonitrile (PhCN), and propylene carbonate (PC)-and substrate concentration on the yield of the coumarin. It was found that the most unsubstituted substrate (1a) afforded the highest yield (41%) of the desired coumarin in a DMF-TBABF(4) medium. A mechanistic scheme is proposed to account for the formation of the coumarin. Furthermore, the only other products seen in these reductions are 2-substituted phenols, which are precursors for synthesis of the various substrates. (C) 2014 The Electrochemical Society. All rights reserved.DOI:10.1149/2.0551412jes
文献信息
-
Pd<sup>II</sup>-Catalyzed Reaction of Phenols with Propiolic Esters. A Single-Step Synthesis of Coumarins作者:Tsugio Kitamura、Kiyomi Yamamoto、Masashi Kotani、Juzo Oyamada、Chengguo Jia、Yuzo FujiwaraDOI:10.1246/bcsj.76.1889日期:2003.10The intermolecular reaction of phenols with propiolic esters in TFA in the presence of a Pd(OAc)2 catalyst, affording coumarin derivatives, is described. An exclusive formation of 5,6,7-trimethoxy-...
-
METHOD OF IMPROVING STABILITY OF SWEET ENHANCER AND COMPOSITION CONTAINING STABILIZED SWEET ENHANCER申请人:TACHDJIAN Catherine公开号:US20120041078A1公开(公告)日:2012-02-16The present invention includes methods of stabilizing one or more sweet enhancers when they are exposed to a light source as well as liquid compositions containing one or more sweet enhancers and one or more photostabilizers.本发明包括在甜味增强剂暴露于光源时稳定一个或多个甜味增强剂的方法,以及包含一个或多个甜味增强剂和一个或多个光稳定剂的液体组合物。
-
Direct Synthesis of Coumarins by Pd(II)-Catalyzed Reaction of Alkoxyphenols and Alkynoates作者:Juzo Oyamada、Chengguo Jia、Yuzo Fujiwara、Tsugio KitamuraDOI:10.1246/cl.2002.380日期:2002.3Reaction of alkoxyphenols and alkynoates in the presence of a catalytic amount of Pd(OAc)2 in trifluoroacetic acid at room temperature gave coumarin derivatives in high yields. This procedure provides a convenient method for direct synthesis of coumarin derivatives under very mild conditions.
-
Cascade Synthesis of 3-Alkenylcoumarins by Palladium-catalyzed Reaction of Phenols and Ethyl Propiolate作者:Tsugio Kitamura、Kotaro Tatemoto、Mariko Sakai、Juzo OyamadaDOI:10.1246/cl.2012.705日期:2012.7.5A highly effective cascade process giving 3-alkenylcoumarins is furnished by a series of reactions involving pallada-arylation of ethyl propiolate with phenols, intramolecular transesterification t...
-
Total Synthesis of (±)–Exotine B作者:Bichu Cheng、Giulio Volpin、Johannes Morstein、Dirk TraunerDOI:10.1021/acs.orglett.8b01817日期:2018.7.20heterodimeric indole/coumarin natural product exotine B was synthesized for the first time. The carbon skeleton of the natural product was formed rapidly by a palladium-catalyzed Suzuki cross-coupling reaction and a gallium-catalyzed three-component [4 + 3] cycloaddition reaction. An alternative biosynthesis of exotine B is proposed based on the total synthesis. Improved syntheses of coumarin natural products
表征谱图
-
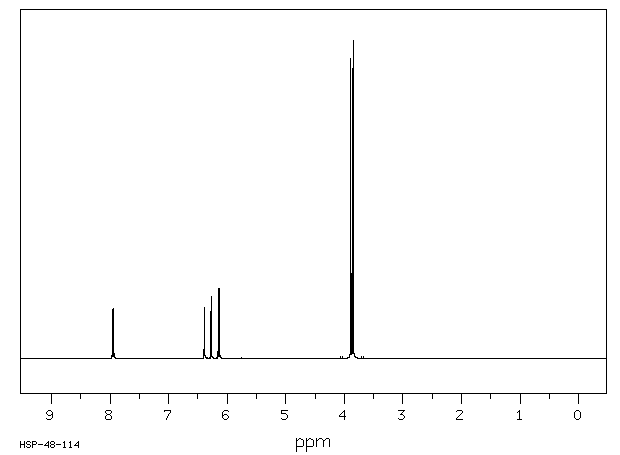
氢谱1HNMR
-
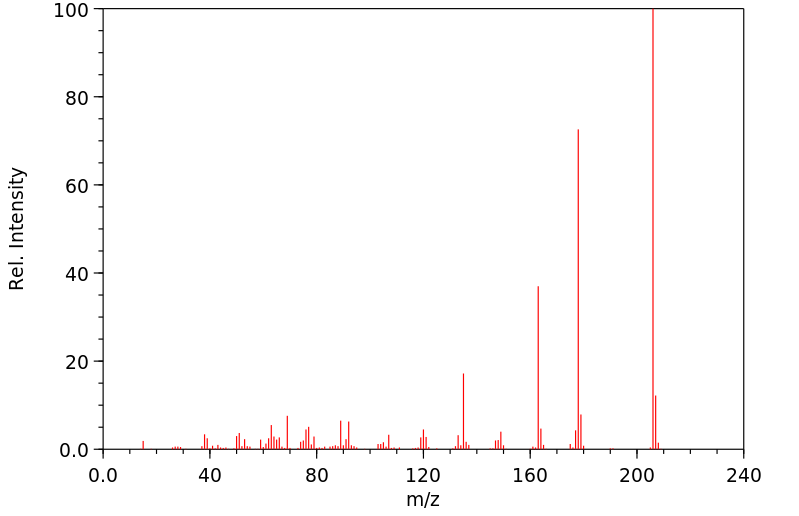
质谱MS
-
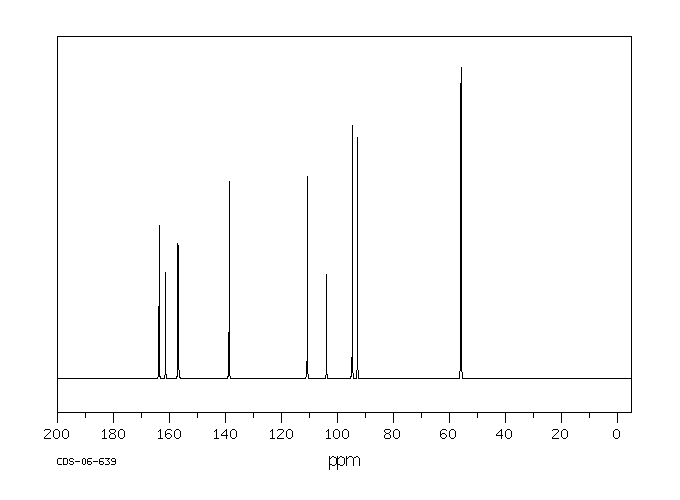
碳谱13CNMR
-
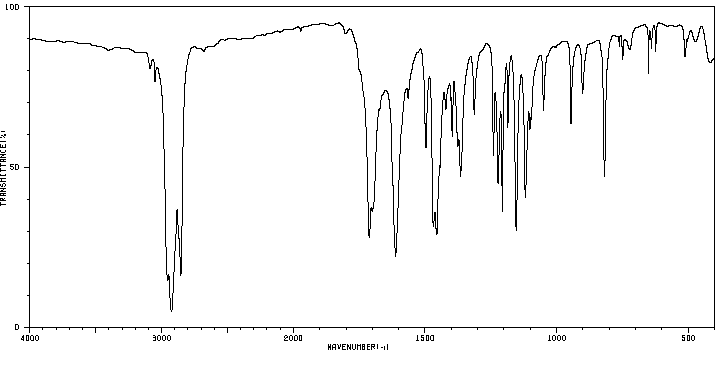
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯