4,4,4-三氟巴豆酸乙酯 | 25597-16-4
中文名称
4,4,4-三氟巴豆酸乙酯
中文别名
4,4,4-三氟-2-丁烯酸乙酯;4,4,4-三氟丁烯酸乙酯;4,4,4,-三氟巴豆酸乙酯
英文名称
Ethyl 4,4,4-trifluorocrotonate
英文别名
ethyl (E)-4,4,4-trifluorobut-2-enoate;(E)-4,4,4-trifluorobut-2-enoic acid ethyl ester;ethyl (E)-4,4,4-trifluorocrotonate
CAS
25597-16-4
化学式
C6H7F3O2
mdl
MFCD00009903
分子量
168.116
InChiKey
ZKRJCMKLCDWROR-ONEGZZNKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:114-115 °C(lit.)
-
密度:1.125 g/mL at 25 °C(lit.)
-
闪点:78 °F
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:11
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:5
安全信息
-
危险等级:3
-
危险品标志:Xi
-
安全说明:S16,S26,S27,S28,S36/37/39,S37/39
-
危险类别码:R10,R36/37/38
-
WGK Germany:3
-
海关编码:2916190090
-
危险品运输编号:UN 3272 3/PG 3
-
危险类别:3
-
包装等级:III
-
储存条件:保持贮藏器密封,并将其放入一个紧密封装的容器中。应将它存放在阴凉、干燥的地方。
SDS
| Name: | Ethyl 4 4 4-Trifluorocrotonate 98% Material Safety Data Sheet |
| Synonym: | Ethyl 4,4,4-Trifluoro-Trans-2-Butenoate |
| CAS: | 25597-16-4 |
Synonym:Ethyl 4,4,4-Trifluoro-Trans-2-Butenoate
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 25597-16-4 | Ethyl 4,4,4-Trifluorocrotonate | 98% | unlisted |
Risk Phrases: 10 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Irritating to eyes, respiratory system and skin.Lachrymator (substance which increases the flow of tears).The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation. Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Vapors may cause dizziness or suffocation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame.
Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 25597-16-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 114 - 115 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 25 deg C ( 77.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.1250g/cm3
Molecular Formula: C6h7fo2
Molecular Weight: 168.12
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 25597-16-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 4,4,4-Trifluorocrotonate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 10 Flammable.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 37 Wear suitable gloves.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 25597-16-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 25597-16-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 25597-16-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
4,4,4-三氟巴豆酸乙酯是一种中间体,广泛应用于合成各种药物活性分子和农产品。此外,在亲核试剂的存在下,它还可以用于合成新型氨基三氟甲基环丙烷羧酸衍生物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4,4,4-trifluorobut-2-enoic acid —— C4H3F3O2 140.062 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl (Z)-4,4,4-trifluorobut-2-enoate —— C6H7F3O2 168.116 4,4,4-三氟丁-2-烯酸 (E)-4,4,4-trifluorobut-2-enoate 406-94-0 C4H3F3O2 140.062 2-甲基-4,4,4-三氟巴豆酸乙酯 Ethyl (2E)-2-methyl-4,4,4-trifluorobutenoate 128227-97-4 C7H9F3O2 182.142 —— (Z/E)-ethyl 2-bromo-4,4,4-trifluorobut-2-enoate 1236144-57-2 C6H6BrF3O2 247.012 (Z)-2-溴-4,4,4-三氟丁烯酸乙酯 ethyl (Z)-2-bromo-4,4,4-trifluorobut-2-enoate 136264-28-3 C6H6BrF3O2 247.012
反应信息
-
作为反应物:描述:4,4,4-三氟巴豆酸乙酯 在 lithium aluminium tetrahydride 、 三氯化铝 作用下, 以 乙醚 为溶剂, 反应 2.0h, 以90%的产率得到4,4,4-三氟-2-丁烯-1-醇参考文献:名称:水中锡和铟介导的烯丙基化反应:β-三氟甲基化高烯丙醇的高立体选择性合成摘要:醛与 4-溴-1,1,1-三氟-2-丁烯在水中的铟介导烯丙基化反应以高产率提供了 β-三氟甲基化的高烯丙醇。在大多数情况下,获得了高区域选择性和优异的非对映选择性。DOI:10.1002/(sici)1099-0690(199908)1999:8<1893::aid-ejoc1893>3.0.co;2-3
-
作为产物:描述:3-羟基-4,4,4-三氟丁酸乙酯 在 磷酸酐 作用下, 反应 1.0h, 以69%的产率得到4,4,4-三氟巴豆酸乙酯参考文献:名称:由氟乙酸酯三步制备丙烯酸β-氟代烷基酯的研究摘要:β-氟烷基丙烯酸酯是有机氟化合物合成的重要组成部分。尽管已知几种β-氟代烷基丙烯酸酯的制备方法,但尚无用于从氟代乙酸酯制备数克这些化合物的通用且直接的实验室规模方法,并且尚未对相关化学方法进行详细研究。现在,我们基于以下内容描述一种优化的三步协议:(1)使用LDA作为碱,将氟乙酸酯与乙酸乙酯进行Claisen型缩合;(2)以甲苯或苯为溶剂,用NaBH 4还原所得的γ-氟-β-酮酯。(3)P 2 O 5促进中间体γ-氟-β-羟基酯的脱水。该方法可提供仅包含氟原子(CF 3,CHF 2,C 2 F 5)的目标化合物的制备有用的产率,而无法以分析纯的形式获得γ-卤代二氟甲基(CClF 2,CBrF 2,CIF 2)丙烯酸酯从脱水步骤中形成。DOI:10.1016/j.tet.2006.12.045
-
作为试剂:描述:4,4,4-三氟巴豆酸乙酯 、 4-tert-butyl-5-chloro-2-hydroxybenzaldehyde 在 4,4,4-三氟巴豆酸乙酯 作用下, 以63的产率得到ethyl 7-(tert-butyl)-6-chloro-2-(trifluoromethyl)-2H-chromene-3-carboxylate参考文献:名称:NO-RELEASING GUANIDINE-CHROMENE CONJUGATES摘要:本公开提供NO释放的胍-色酮共轭物,其结构为公式(I):其中R1、R2、R3、R4、R10和L如详细说明中定义的那样;包含公式(I)中至少一种化合物的制药组合物;以及利用公式(I)中的化合物用于治愈创伤、预防和治疗癌症、治疗光化性角化症、囊性纤维化、痤疮或由精氨酸缺乏介导的疾病的方法。公开号:US20150197500A1
文献信息
-
[EN] SPHINGOSINE-1-PHOSPHATE RECEPTOR AGONISTS<br/>[FR] AGONISTES DES RÉCEPTEURS SPHINGOSINE-1-PHOSPHATE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2011017578A1公开(公告)日:2011-02-10Disclosed are compounds of Formula (I), or a stereoisomer or a pharmaceutically acceptable salt thereof, wherein: A is formula (II) Q is a substituted 5-membered monocyclic heteroaryl group; W is CH2, O, or NH; and R1, R2, R3, R4, R5, R6, m, n, t, and x are defined herein. Also disclosed are methods of using such compounds as selective agonists for G protein-coupled receptor S1P1, and pharmaceutical compositions comprising such compounds. These compounds are useful in treating, preventing, or slowing the progression of diseases or disorders in a variety of therapeutic areas, such as autoimmune diseases and vascular disease.揭示了Formula (I)的化合物,或其立体异构体或药用可接受的盐,其中:A为Formula (II),Q为取代的5-成员单环杂芳基团;W为CH2、O或NH;而R1、R2、R3、R4、R5、R6、m、n、t和x在此处有定义。还揭示了将这些化合物用作G蛋白偶联受体S1P1的选择性激动剂的方法,以及包含这些化合物的药物组合物。这些化合物在治疗、预防或减缓多种治疗领域的疾病或紊乱方面具有用处,如自身免疫疾病和血管疾病。
-
Herbicidal glutaramic acids and derivatives申请人:Rohm and Haas Company公开号:US05238908A1公开(公告)日:1993-08-24This invention relates to glutaramic acids and derivatives exhibiting herbicidal activity having the structure ##STR1## wherein A is a carboxylic acid or a derivative thereof, D is CH or N, and R, R.sup.1, R.sup.2, T, X, Y, and Z are as defined within, compositions containing these compounds and methods of controlling weeds with these compounds.
-
[EN] 4 - (5 - ISOXAZOLYL OR 5 - PYRRAZOLYL -1,2,4- OXADIAZOL - 3 - YL) -MANDELIC ACID AMIDES AS SPHINGOSIN- 1 - PHOSPHATE 1 RRECEPTOR AGONISTS<br/>[FR] AMIDES D'ACIDE 4-(5-ISOXAZOLYL OU 5-PYRAZOLYL-1,2,4-OXADIAZOL-3-YL)-MANDÉLIQUE COMME AGONISTES DU RÉCEPTEUR DE LA SPHINGOSINE-1-PHOSPHATE DE TYPE 1申请人:BRISTOL MYERS SQUIBB CO公开号:WO2011133734A1公开(公告)日:2011-10-27Disclosed are compounds of Formula (I) or stereoisomers, salts, or prodrugs thereof, wherein: Q is, or R1 is phenyl substituted with zero to 3 substituents; and R1, R2, R3, R4, R5, and G are defined herein. Also disclosed are methods of using such compounds as selective agonists for G protein-coupled receptor S1P1, and pharmaceutical compositions comprising such compounds. These compounds are useful in treating, preventing, or slowing the progression of diseases or disorders in a variety of therapeutic areas, such as autoimmune diseases and vascular disease.揭示了Formula (I)的化合物或其立体异构体、盐或前药,其中:Q是,或者R1是苯基,其上取代基为零至3个;而R1、R2、R3、R4、R5和G在此处有定义。还揭示了将这些化合物用作G蛋白偶联受体S1P1的选择性激动剂的方法,以及包含这些化合物的药物组合物。这些化合物在治疗、预防或减缓多种治疗领域的疾病或紊乱方面具有用处,如自身免疫疾病和血管疾病。
-
Bicyclic-Fused Heteroaryl or Aryl Compounds申请人:Pfizer Inc.公开号:US20150284405A1公开(公告)日:2015-10-08Compounds, tautomers and pharmaceutically acceptable salts of the compounds are disclosed, wherein the compounds have the structure of Formula Ia, as defined in the specification. Corresponding pharmaceutical compositions, methods of treatment, methods of synthesis, and intermediates are also disclosed.所述化合物的异构体和药学上可接受的盐已被披露,其中所述化合物具有如规范中定义的Ia式结构。相应的药物组合物、治疗方法、合成方法和中间体也已被披露。
-
Antibody drug conjugate, intermediate, preparation method, pharmaceutical composition and uses thereof申请人:XDCEXPLORER (SHANGHAI) CO., LTD.公开号:US10449258B2公开(公告)日:2019-10-22Disclosed are an antibody drug conjugate IB, which uses ether linkages for connection, and improves the water solubility, stability and cytotoxicity in vivo and in intro, and an intermediate, a pharmaceutical composition, and uses of the antibody drug conjugate. The antibody drug conjugate has simple synthetic steps and a high yield.
表征谱图
-
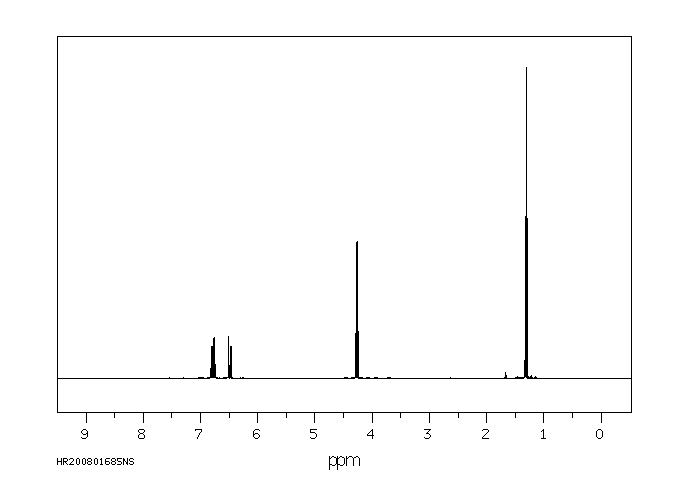
氢谱1HNMR
-
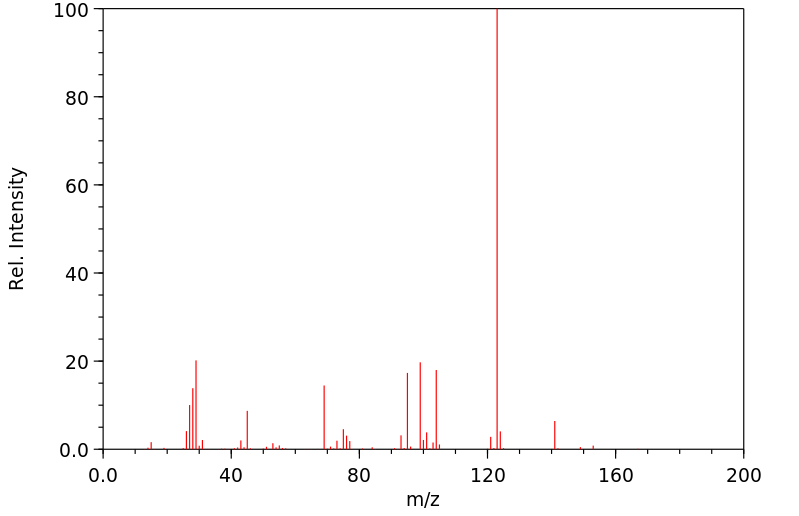
质谱MS
-
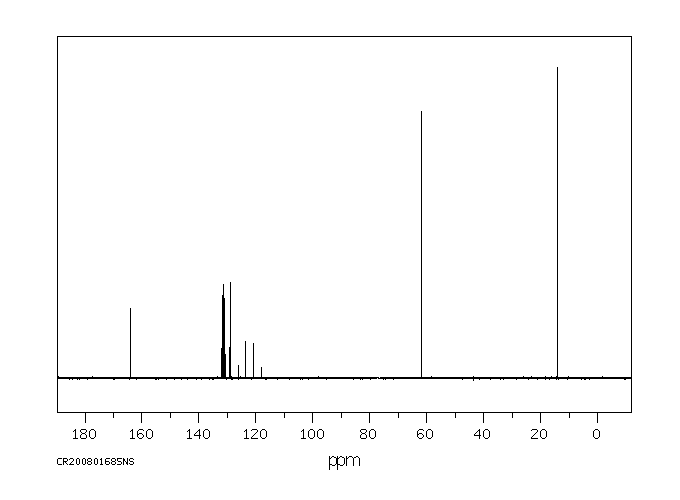
碳谱13CNMR
-
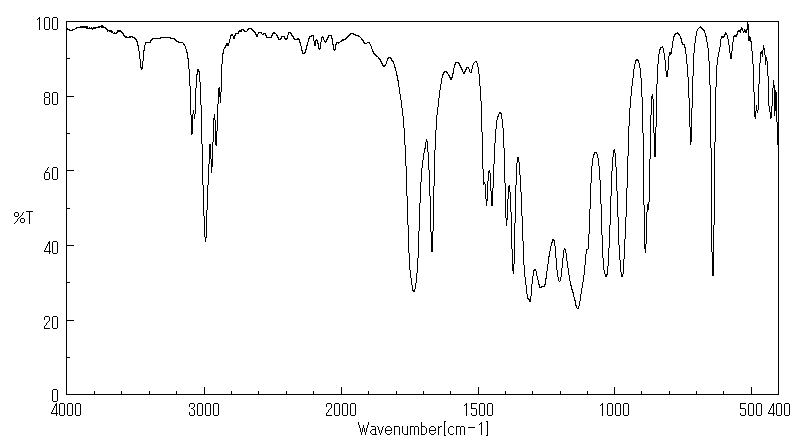
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯