N,N-二氰乙基苯胺 | 1555-66-4
中文名称
N,N-二氰乙基苯胺
中文别名
对异丁基苯乙酸;布芬酸
英文名称
N,N-bis(2-cyanoethyl)aniline
英文别名
N,N-dicyanoethylaniline;N,N-bis-β-cyanoethylaniline;N,N-Bis(cyanoethyl)aniline;3-[N-(2-cyanoethyl)anilino]propanenitrile
CAS
1555-66-4
化学式
C12H13N3
mdl
MFCD00019855
分子量
199.255
InChiKey
NSVHSAUVIFTVPN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:81-84 °C(lit.)
-
沸点:326.78°C (rough estimate)
-
密度:1.0222 (rough estimate)
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解,没有已知的危险反应。应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:50.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
安全说明:S26,S36
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
| Name: | n n-bis(Cyanoethyl)aniline 95% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1555-66-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1555-66-4 | N,N-Bis(cyanoethyl)aniline | 95% | 216-306-4 |
Risk Phrases: 20/21/22 40 50 48/23/24/25
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Limited evidence of a carcinogenic effect. Very toxic to aquatic organisms. Toxic : danger of serious damage to health by prolonged exposure through inhalation, contact with skin and if swallowed.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. May cause respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1555-66-4: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light beige
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 80 - 88 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C12H13N3
Molecular Weight: 199.25
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, acids, acetic anhydride, acid chlorides, carbon dioxide.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1555-66-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N,N-Bis(cyanoethyl)aniline - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: 3
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: 3
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T N
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 40 Limited evidence of a carcinogenic effect.
R 48/23/24/25 Toxic : danger of serious damage to
health by prolonged exposure through inhalation,
contact with skin and if swallowed.
R 50 Very toxic to aquatic organisms.
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
S 36/37 Wear suitable protective clothing and
gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
S 61 Avoid release to the environment. Refer to
special instructions/safety data sheets.
WGK (Water Danger/Protection)
CAS# 1555-66-4: No information available.
Canada
CAS# 1555-66-4 is listed on Canada's NDSL List.
CAS# 1555-66-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1555-66-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二羟乙基苯胺 2,2'-(phenylimino)bis[ethanol] 120-07-0 C10H15NO2 181.235 N,N-双(2-氯乙基)苯胺 aniline mustard 553-27-5 C10H13Cl2N 218.126 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,3'-(4-bromo-phenylimino)-di-propionitrile 99990-40-6 C12H12BrN3 278.151 —— bis(aminopropyl)aniline 1555-72-2 C12H21N3 207.319 3,3'-[(4-甲酰基苯基)亚氨基]二丙腈 4-[di-2-cyanoethyl-amino]benzaldehyde 17354-79-9 C13H13N3O 227.266 —— N-ethoxycarbonyl-N-phenyl-β-alanine 52766-37-7 C12H15NO4 237.255
反应信息
-
作为反应物:描述:参考文献:名称:芳族伯胺的双-2-氰基乙基衍生物的制备,并将其转化为1:6-二酮甲柔啶。第三部分摘要:DOI:10.1039/jr9540000651
-
作为产物:描述:参考文献:名称:334. 3-乙基吡啶和2:3-呋喃基(2':3')吡啶的某些衍生物摘要:DOI:10.1039/jr9340001536
文献信息
-
Triethylammonium acetate (TEAA): a recyclable inexpensive ionic liquid promotes the chemoselective aza- and thia-Michael reactions作者:Akhilesh K. Verma、Pankaj Attri、Varun Chopra、Rakesh K. Tiwari、Ramesh ChandraDOI:10.1007/s00706-008-0886-4日期:2008.9A new, highly efficient, inexpensive, recyclable, mild, convenient, and green protocol for chemoselective aza/thia- Michael addition reactions of amines/thiols to α,β - unsaturated compounds using triethylammonium acetate ( TEAA ) ionic liquid was developed. The catalyst can be recycled ten times and obviate the need for toxic and expensive catalysts.
-
Regioselective biotransformations of dinitriles using Rhodococcus sp. AJ2701作者:Otto Meth-Cohn、Mei-Xiang WangDOI:10.1039/a703904b日期:——or γ-sulfur is present. Hydrolysis of N,N-bis(2-cyanoethyl)anilines 4h–j takes place slowly affording exclusively the monoacids 5h–j while the monocyano amides 5o–p are obtained as the sole isolable product from rapid hydrolysis of the corresponding N,N-bis(2-cyanomethyl)butylamine 4o and N,N-bis(3-cyanopropyl)butylamine 4p. Higher homologues of arylimino- and butylimino-dinitriles are inert to enzymatic使用Rhodococcus sp在非常温和的条件下选择性地水解了多种二腈。AJ270。脂族二腈NC [CH 2 ] n CN 1进行区域选择性水解,得到的单官能酸2在腈官能团之间最多具有4个亚甲基,而n > 4的单酸2获得的二酸3的收率很好。当将氧置于氰基的β,γ或δ或β-或γ-上时,带有醚键或硫化物键的二腈NC [CH 2 ] n X [CH 2 ] n CN 4被有效地转化为一元酸5。存在硫。N,N的水解双(2-氰乙基)苯胺的4H-j取而monocyano酰胺0-50 p如从相应的快速水解的唯一可分离产物获得缓慢发生,得到专门的一元酸5H-J Ñ,Ñ双(2-氰基)丁胺4o和N,N-双(3-氰基丙基)丁胺4p。芳基-和丁基亚氨基-二腈的较高同系物对酶促水解是惰性的。多种其它脂族二腈的已被容易地转化成单酸以良好至优异的产率,除了ö -phenylenediacetonitrile其给出ø
-
Addition of amines and phenols to acrylonitrile derivatives catalyzed by the POCOP-type pincer complex [{κP,κC,κP-2,6-(i-Pr2PO)2C6H3}Ni(NCMe)][OSO2CF3]作者:Xavier Lefèvre、Guillaume Durieux、Stéphanie Lesturgez、Davit ZargarianDOI:10.1016/j.molcata.2010.11.010日期:2011.2anti-Markovnikov addition of nucleophiles to activated olefins. The catalyzed additions of aliphatic amines to acrylonitrile, methacrylonitrile, and crotonitrile proceed at room temperature and give quantitative yields of products resulting from the formation of C–N bonds. On the other hand, aromatic amines or alcohols are completely inert toward methacrylonitrile and crotonitrile, and much less reactive钳型配合物[κ P,κ Ç,κ P -2,6-(我-Pr 2 PO)2 C ^ 6 ħ 3 }的Ni(NCMe)] [OSO 2 CF 3 ](1)可以用作亲核试剂向活性烯烃的区域选择性,反马尔科夫尼科夫加成的前催化剂。脂肪胺在丙烯腈,甲基丙烯腈和巴豆腈中的催化加成反应在室温下进行,并由于形成了C–N键而产生定量的产物收率。另一方面,芳族胺或醇对甲基丙烯腈和巴豆腈是完全惰性的,对丙烯腈的反应性小得多,需要添加碱,加热和延长的反应时间才能获得良好的收率。1的催化反应性据认为是由于配位乙腈的取代不稳定性所致,该取代基使得烯烃底物中腈部分具有竞争性配位;这种结合增强了CC部分的亲电子性,使它们更易于受到亲核试剂的攻击。在某些情况下,RCN→Ni结合会导致双键异构化/迁移(烯丙基氰化物)或亲核体在腈部分(肉桂腈和4-氰基苯乙烯)的侵蚀。吗啉与1在60°C下反应导致形成formation衍生物2,该X衍生物2已通过X射线晶体学表征。
-
Monoazo dyes which are free from water-solubilizing groups and which申请人:Zeneca Limited公开号:US05739299A1公开(公告)日:1998-04-14A process for coloring a synthetic textile material or fibre blend thereof which comprises applying to the synthetic textile material a compound or mixture thereof, which is free from water solubilizing groups, of Formula (1): A--N.dbd.N--D Formula (1) wherein A and D each independently is an optionally substituted heterocyclic or carbocyclic group and at least one of A or D carries directly at least one --SO.sub.2 F group or carries a substituent to which at least one --SO.sub.2 F group is attached except for 4-(4-fluorosulphonylphenylazo)-N,N-dimethylaniline, provided that one of A or D is not 3,5-difluorosulphonylthien-2-yl, optionally substituted 1-phenyl-pyrazol-4-yl-5-one or ##STR1## or that one of A or D does not carry an --NCH.sub.2 CH(OH)CH.sub.2 Cl, --NCOCH.sub.2 Cl or --NCH.sub.2 CH.sub.2 SO.sub.2 F substituent. The presence of one or more --SO.sub.2 F groups in a dye molecule generally improves the properties of that dye and confers surprisingly good wet fastness and light fastness properties.一种用于给合成纺织材料或其纤维混合物着色的方法,包括向合成纺织材料施加一个不含水溶解基团的化合物或其混合物,其化学式为(1):A--N.dbd.N--D 化学式(1)其中A和D各自独立地是一个可选择地取代的杂环或碳环基团,且A或D中至少一个直接携带至少一个--SO.sub.2 F基团,或携带一个取代基,至少一个--SO.sub.2 F基团附着在该取代基上,但不包括4-(4-氟磺酰基苯基偶氮)-N,N-二甲基苯胺,条件是A或D中的一个不是3,5-二氟磺酰基噻吩-2-基,可选择地取代的1-苯基吡唑-4-基-5-酮或##STR1##,或者A或D中的一个不携带--NCH.sub.2 CH(OH)CH.sub.2 Cl,--NCOCH.sub.2 Cl或--NCH.sub.2 CH.sub.2 SO.sub.2 F取代基。染料分子中存在一个或多个--SO.sub.2 F基团通常会改善该染料的性能,并赋予出乎意料的良好湿度牢度和光牢度性能。
-
Copolymerizable methine and anthraquinone compounds and articles containing them申请人:Pearson Clay Jason公开号:US20060115516A1公开(公告)日:2006-06-01This invention relates to polymerizable ultraviolet light absorbers and yellow colorants and their use in ophthalmic lenses. In particular, this invention relates to polymerizable ultraviolet light absorbing methine compounds and yellow compounds of the methine and anthraquinone classes that block ultraviolet light and/or violet-blue light transmission through ophthalmic lenses.
表征谱图
-
氢谱1HNMR
-
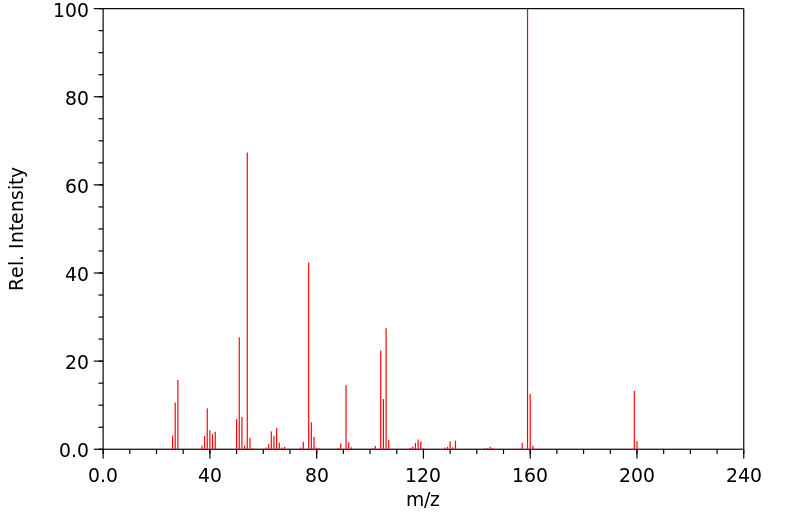
质谱MS
-
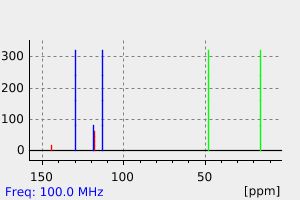
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷