2,5-二甲基-2-己烯 | 3404-78-2
中文名称
2,5-二甲基-2-己烯
中文别名
——
英文名称
2,5-dimethylhex-2-ene
英文别名
2,5-dimethyl-2-hexene
CAS
3404-78-2
化学式
C8H16
mdl
MFCD00026436
分子量
112.215
InChiKey
VFZIUYUUQFYZBR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-103.01°C (estimate)
-
沸点:113 °C
-
密度:0,72 g/cm3
-
闪点:11°C(lit.)
-
介电常数:2.4300000000000002
-
保留指数:762;760;761;768;759;760;762;762;762;762;762;751;759;750
-
稳定性/保质期:
- 常温常压下稳定。
- 存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品运输编号:UN 1216
-
海关编码:2901299090
-
安全说明:S16
-
危险类别码:R10
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
2,5-Dimethyl-2-hexene
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,5-Dimethyl-2-hexene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 2
Flammable liquids
HEALTH HAZARDS
Category 1
Aspiration hazard
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Highly flammable liquid and vapour
May be fatal if swallowed and enters airways
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
induce vomiting.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,5-Dimethyl-2-hexene
>95.0%(GC)
Percent:
CAS Number: 3404-78-2
Chemical Formula: C8H16
2,5-Dimethyl-2-hexene
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
2,5-Dimethyl-2-hexene
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Almost colorless
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 113°C
Flash point: 11°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.72
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
2,5-Dimethyl-2-hexene
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system but exert extra care in igniting as this material is
highly flammable. Observe all federal, state and local regulations when disposing of the substance
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
3295
UN-No:
Proper shipping name: Hydrocarbons, liquid, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,5-Dimethyl-2-hexene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 2
Flammable liquids
HEALTH HAZARDS
Category 1
Aspiration hazard
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Highly flammable liquid and vapour
May be fatal if swallowed and enters airways
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
induce vomiting.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,5-Dimethyl-2-hexene
>95.0%(GC)
Percent:
CAS Number: 3404-78-2
Chemical Formula: C8H16
2,5-Dimethyl-2-hexene
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
2,5-Dimethyl-2-hexene
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Almost colorless
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 113°C
Flash point: 11°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.72
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
2,5-Dimethyl-2-hexene
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system but exert extra care in igniting as this material is
highly flammable. Observe all federal, state and local regulations when disposing of the substance
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
3295
UN-No:
Proper shipping name: Hydrocarbons, liquid, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Tuot, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1933, vol. 197, p. 1436摘要:DOI:
-
作为产物:描述:反式-2,5-二甲基-3-己烯 在 calcium amide 作用下, 生成 2,5-二甲基-2-己烯参考文献:名称:Gostunskaja et al., Doklady Akademii Nauk SSSR, 1956, vol. 108, p. 473摘要:DOI:
文献信息
-
Direct Synthesis of 1,4-Diols from Alkenes by Iron-Catalyzed Aerobic Hydration and CH Hydroxylation作者:Takuma Hashimoto、Daisuke Hirose、Tsuyoshi TaniguchiDOI:10.1002/anie.201308675日期:2014.3.3Various 1,4‐diols are easily accessible from alkenes through iron‐catalyzed aerobic hydration. The reaction system consists of a user‐friendly iron phthalocyanine complex, sodium borohydride, and molecular oxygen. Furthermore, the effect of additional ligands on the iron complex was examined for a model reaction. The second hydroxy group is installed by direct C(sp3)H oxygenation, which is based on
-
Direct Cross-Coupling of Allylic C(sp<sup>3</sup> )−H Bonds with Aryl- and Vinylbromides by Combined Nickel and Visible-Light Catalysis作者:Long Huang、Magnus RuepingDOI:10.1002/anie.201805118日期:2018.8.6An efficient protocol for the direct allylic C(sp3)−H bond activation of unactivated tri‐ and tetrasubstituted alkenes and their functionalization with aryl‐ and vinylbromides by nickel and visible‐light photocatalysis has been developed. The method allows C(sp2)−C(sp3) formation under mild reaction conditions with good functional‐group tolerance and excellent regioselectivity.
-
Enantioselective [2 + 2] Cycloaddition of Unactivated Alkenes with α-Acyloxyacroleins Catalyzed by Chiral Organoammonium Salts作者:Kazuaki Ishihara、Kazuhiko NakanoDOI:10.1021/ja073435w日期:2007.7.1enantioselective [2 + 2] cycloaddition reaction of unactivated alkenes with α-acyloxyacroleins to give optically active 1-acyloxycyclobutanecarbaldehydes and subsequent ring expansion to give optically active 2-hydroxycyclopentanone derivatives. Organoammonium salt, H-L-Phe-L-Leu-N(CH2CH2)2-reduced triamine•2.6HNTf2, is a highly effective chiral catalyst for the above enantioselective [2 + 2] cycloaddition. Thus
-
Direct studies of 1,1-diazenes. Syntheses, infrared and electronic spectra, and kinetics of the thermal decomposition of N-(2,2,6,6-tetramethylpiperidyl)nitrene and N-(2,2,5,5-tetramethylpyrrolidyl)nitrene作者:William D. Hinsberg、Peter G. Schultz、Peter B. DervanDOI:10.1021/ja00367a020日期:1982.2The syntheses, direct spectroscopic observation, and kinetics of thermal decomposition of the persistent 1,1-diazenes, N-(2,2,6,6-tetramethylpiperidyl)nitrene (4) and N-(2,2,5,5-tetramethylpyrrolidyl)nitrene (5) are reported. The electronic absorption spectrum of 4 at -78 °C reveals a structured absorption for the n,π* transition: λmax= 543 nm, λ_(0,0) = 620 nm, and emax = 18 ± 3 in Et_2O; λmax= 541持久性 1,1-二氮烯、N-(2,2,6,6-四甲基哌啶基)硝烯 (4) 和 N-(2,2,5,5-) 的合成、直接光谱观察和热分解动力学报道了四甲基吡咯烷基)氮(5)。-78 °C 下 4 的电子吸收光谱揭示了 n,π* 跃迁的结构化吸收:λmax= 543 nm,λ_(0,0) = 620 nm,Et_2O 中的 emax = 18 ± 3;λmax= 541 nm 和 λ_(0,0) = 610 nm 在 CH_2Cl_2 中;在 i-PrOH 中,λmax= 526 nm 和 λ_(0,0) = 592 nm。4 的红外光谱在 1595 cm^(-1)(R_2^(14) = ^(14)N 拉伸) 处显示出强吸收,并提供证据表明 1,1-二氮烯 4 具有相当大的 N=N 双键特征在地面状态。5 的红外光谱在 1638 cm^(-1)(R_2^(14) = ^(14)N 拉伸) 处显示
-
Superelectrophilic Fe(III)–Ion Pairs as Stronger Lewis Acid Catalysts for (<i>E</i>)-Selective Intermolecular Carbonyl–Olefin Metathesis作者:Haley Albright、Hannah L. Vonesh、Corinna S. SchindlerDOI:10.1021/acs.orglett.0c00917日期:2020.4.17intermolecular carbonyl–olefin metathesis reaction is described that relies on superelectrophilic Fe(III)-based ion pairs as stronger Lewis acid catalysts. This new catalytic system enables selective access to (E)-olefins as carbonyl–olefin metathesis products. Mechanistic investigations suggest the regioselective formation and stereospecific fragmentation of intermediate oxetanes to be the origin of this
表征谱图
-
氢谱1HNMR
-
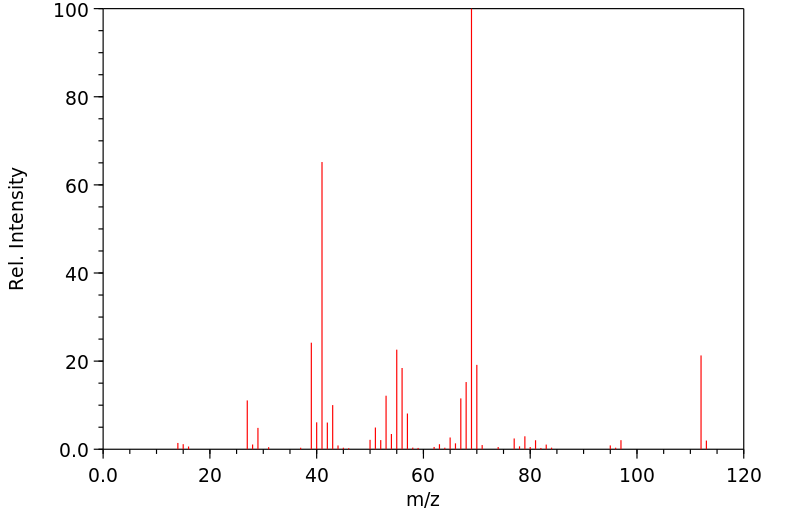
质谱MS
-
碳谱13CNMR
-
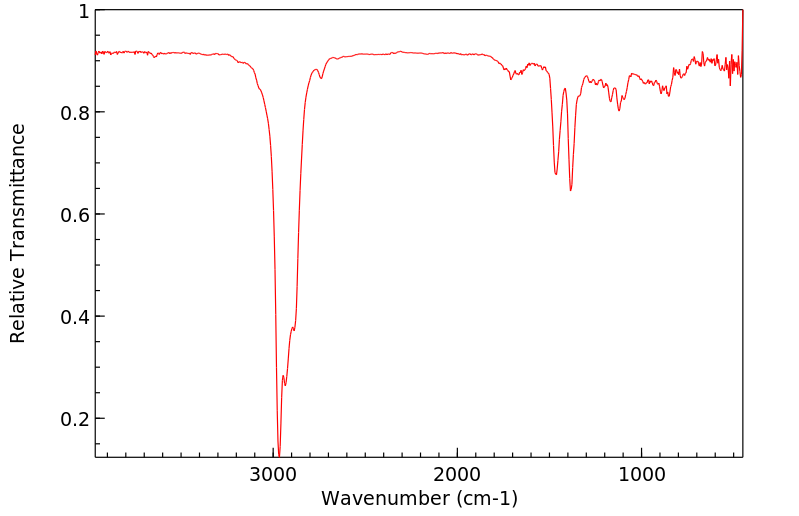
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-