环丙烷-1,2-二羧酸二乙酯 | 20561-09-5
中文名称
环丙烷-1,2-二羧酸二乙酯
中文别名
——
英文名称
diethyl 1,2-cyclopropanedicarboxylate
英文别名
diethyl (cis,trans)-1,2-cyclopropanedicarboxylate;diethyl cyclopropane-1,2-dicarboxylate
CAS
20561-09-5
化学式
C9H14O4
mdl
MFCD00001286
分子量
186.208
InChiKey
SXLDHZFJMXLFJU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:70-75 °C (1 mmHg)
-
密度:1.06
-
闪点:98 °C
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:13
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.777
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S24/25
-
海关编码:2917209090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:储存条件:2-8°C,干燥密封。
SDS
| Name: | Diethyl 1 2-Cyclopropanedicarboxylate 97% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 20561-09-5 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 20561-09-5 | Diethyl 1,2-Cyclopropanedicarboxylate | 97 | 243-878-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Vapors may cause dizziness or suffocation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation.
Storage:
Keep away from heat, sparks, and flame. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 20561-09-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 70.0 - 75.0 deg C @ 1.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 98 deg C ( 208.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.0610g/cm3
Molecular Formula: C9H14O4
Molecular Weight: 186.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 20561-09-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diethyl 1,2-Cyclopropanedicarboxylate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 20561-09-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 20561-09-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 20561-09-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 顺式-环丙烷-1,2-二羧酸二乙酯 cis-cyclopropane-1,2-dicarboxylic acid diethyl ester 710-43-0 C9H14O4 186.208 1,2-环丙烷二羧酸-1-乙酯 trans-2-(ethoxycarbonyl)cyclopropane-1-carboxylic acid 167113-73-7 C7H10O4 158.154 顺-1,2-环丙烷二羧酸二甲酯 dimethyl cis-cyclopropane-1,2-dicarboxylate 826-34-6 C7H10O4 158.154 —— cis-1,2-cyclopropanedicarboxylic acid 696-74-2 C5H6O4 130.1 1,2-环丙烷二羧酸 cyclopropane-1,2-dicarboxylic acid 1489-58-3 C5H6O4 130.1 3-氧杂二环[3.1.0]己烷-2,4-二酮 3-oxabicyclo[3.1.0]hexane-2,4-dione 5617-74-3 C5H4O3 112.085 —— cis-1,2-cyclopropanedicarboxylic acid anhydride 5617-74-3 C5H4O3 112.085
反应信息
-
作为反应物:描述:环丙烷-1,2-二羧酸二乙酯 在 水 、 乙酸酐 、 sodium hydroxide 作用下, 以 水 为溶剂, 反应 15.0h, 生成 cis-1,2-cyclopropanedicarboxylic acid参考文献:名称:大规模制备顺式环丙烷二胺二盐酸盐的高效改进方法摘要:以100克规模开发了一种有效且改进的制备顺式环丙戊二胺二盐酸盐的方法。该方法的关键步骤是通过形成环酸酐,由顺式和反式异构体的混合物制备顺式环丙烷-1,2-二羧酸。整个过程和所有过程都是经济的,工业上可靠的并且易于扩大规模。DOI:10.2174/1570178612666150907204813
-
作为产物:描述:戊二酸二乙酯 在 copper(II) bis(trifluoromethanesulfonate) 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 为溶剂, 反应 30.0h, 以62%的产率得到环丙烷-1,2-二羧酸二乙酯参考文献:名称:Kobayashi, Yoshiro; Taguchi, Takeo; Morikawa, Tsutomu, Chemical and pharmaceutical bulletin, 1980, vol. 28, # 1, p. 262 - 267摘要:DOI:
文献信息
-
[EN] HETEROCYCLIC COMPOUNDS AND THEIR USE AS RETINOID-RELATED ORPHAN RECEPTOR (ROR) GAMMA-T INHIBITORS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES ET LEUR UTILISATION EN TANT QU'INHIBITEURS GAMMA-T DU RÉCEPTEUR ORPHELIN APPARENTÉ AUX RÉCEPTEURS DES RÉTINOÏDES (ROR) )申请人:TAKEDA PHARMACEUTICAL公开号:WO2016002968A1公开(公告)日:2016-01-07Provided are heterocyclic compounds having a RORγt inhibitory action represented by the formula (I): wherein each symbol is as defined in the specification, or a salt thereof.提供的是具有RORγt抑制作用的杂环化合物,其由公式(I)表示:其中每个符号如说明书中定义,或其盐。
-
Chemokine receptor binding heterocyclic compounds with enhanced efficacy申请人:——公开号:US20030220341A1公开(公告)日:2003-11-27The invention relates to heterocyclic compounds consisting of a core nitrogen atom surrounded by three pendant groups, wherein two of the three pendant groups are preferably benzimidazolyl methyl and tetrahydroquinolyl, and the third pendant group contains N and optionally contains additional rings. The compounds bind to chemokine receptors, including CXCR4 and CCR5, and demonstrate protective effects against infection of target cells by a human immunodeficiency virus (HIV).
-
Imide derivatives, and their production and use申请人:Sumitomo Pharmaceuticals Company, Ltd.公开号:US05532372A1公开(公告)日:1996-07-02An imide compound of the formula: ##STR1## wherein Z is a group of the formula: ##STR2## in which the substituents are defined herein, and n is an integer of 0 to 1; D is a group of the formula: --(CH.sub.2).sub.p --A--(CH.sub.2).sub.q -- in which A is a non-aromatic hydrocarbon ring optionally bridged with a lower alkylene group or an oxygen atom, said non-aromatic hydrocarbon ring and said lower alkylene group being each optionally substituted with at least one lower alkyl, and p and q are each an integer of 0, 1 or 2; and Ar is an aromatic group, a heterocyclic aromatic group, a benzoyl group, a phenoxy group or a phenylthio group and G is >N--, >CH-- or >COH-- or Ar is a biphenylmethylidene group and G is >C.dbd., all of the above groups being each optionally substituted with at least one of lower alkyl, lower alkoxy and halogen; and its acid addition salts, useful as an antipsycotic agent.公式为:##STR1## 其中 Z 是一个具有以下结构的基团:##STR2## 在其中,取代基在此处定义,n 是 0 到 1 的整数;D 是一个具有以下结构的基团:--(CH.sub.2).sub.p --A--(CH.sub.2).sub.q -- 其中 A 是一个非芳香烃环,可选择地与较低的烷基链或氧原子桥接,所述非芳香烃环和所述较低的烷基链各自可选择地取代至少一个较低的烷基,p 和 q 各自是 0、1 或 2 的整数;Ar 是一个芳香基团,一个杂环芳香基团,一个苯甲酰基团,一个苯氧基团或一个苯硫基团,G 是 >N--,>CH-- 或 >COH-- 或 Ar 是一个联苯甲基亚基团,G 是 >C.dbd.,上述所有基团均可选择地取代至少一个较低烷基、较低烷氧基和卤素;以及其酸盐,作为抗精神病药物。
-
[EN] HETEROCYCLIC INHIBITORS OF GLUTAMINASE<br/>[FR] INHIBITEURS HÉTÉROCYCLIQUES DE GLUTAMINASE申请人:CALITHERA BIOSCIENCES INC公开号:WO2013078123A1公开(公告)日:2013-05-30The invention relates to the heterocyclic compounds of Formula (I) as defined further herein, and pharmaceutical preparations thereof. The invention further relates to methods of treating cancer, immunological or neurological diseases using the heterocyclic compounds of the invention.
-
Halothenoyl-cyclopropane-1-carboxylic acid derivatives申请人:Newron Pharmaceuticals S.p.A.公开号:EP1424333A1公开(公告)日:2004-06-02Compounds of formula (I) wherein R is hydroxy, linear or branched C1-C6 alkoxy, phenoxy, benzyloxy, a group -N(R1R2) wherein R1 is hydrogen, linear or branched C1-C4 alkyl, benzyl, phenyl and R2 is hydrogen or linear or branched C1-C4 alkyl, or R is a glycoside residue or a primary alkoxy residue from ascorbic acid, optionally having one or more hydroxy groups alkylated or acylated by linear or branched C1-C4 alkyl or acyl groups; X is a halogen atom and n 1 or 2 are long lasting inhibitors of kynurenine 3-monooxygenase (KMO) and potent glutamate (GLU) release inhibitors.
表征谱图
-
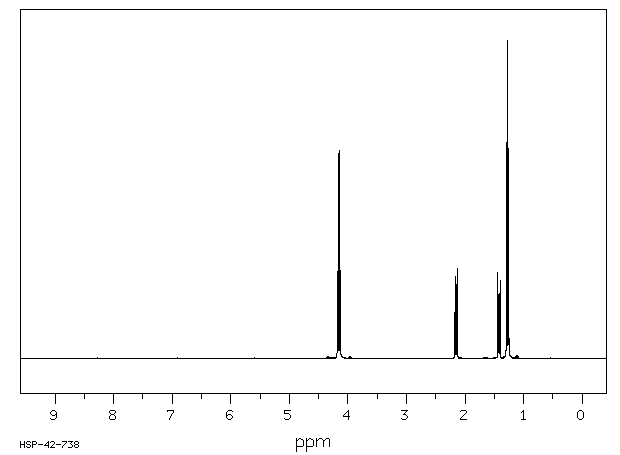
氢谱1HNMR
-
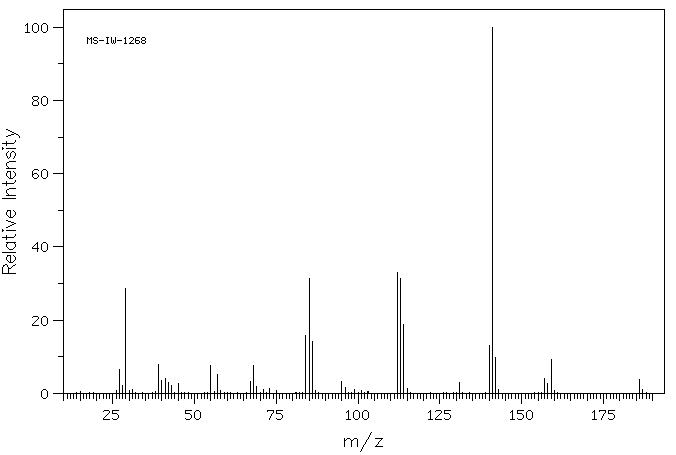
质谱MS
-
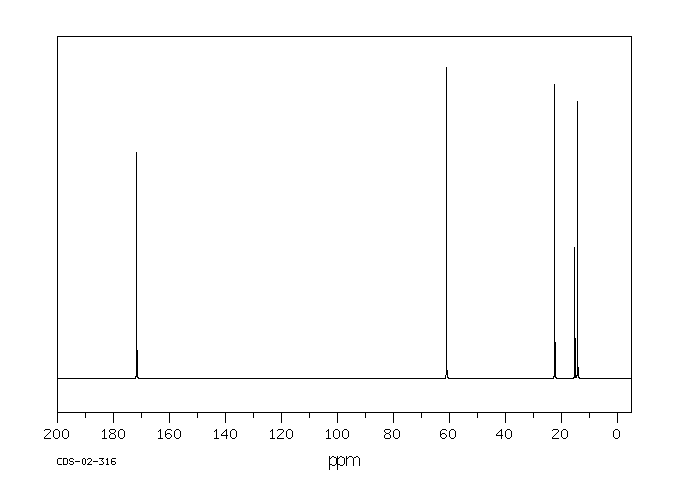
碳谱13CNMR
-
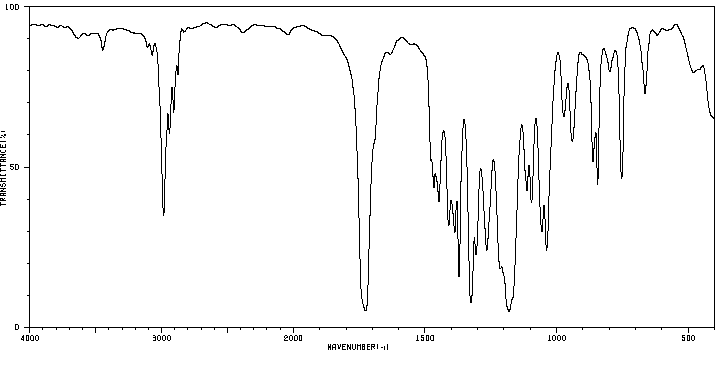
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸