N-乙酰基酪胺 | 1202-66-0
中文名称
N-乙酰基酪胺
中文别名
N-(4-羟基苯乙基)乙酰胺;N-[2-(4-羟基苯基)乙基]乙酰胺
英文名称
N-acetyltyramine
英文别名
N-(4-hydroxyphenylethyl)acetamide;(N-(2-(4-hydroxyphenyl)ethyl)acetamide);N-[2-(4-hydroxyphenyl)ethyl]-acetamide;N-[2-(4-hydroxyphenyl)ethyl]acetamide;2-(p-hydroxyphenyl)ethylacetamide;N-(4-hydroxyphenethyl) acetamide
CAS
1202-66-0
化学式
C10H13NO2
mdl
——
分子量
179.219
InChiKey
ATDWJOOPFDQZNK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:169 °C
-
沸点:424.1±28.0 °C(Predicted)
-
密度:1.122±0.06 g/cm3(Predicted)
-
溶解度:溶于二甲基亚砜
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:49.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2924299090
-
WGK Germany:3
-
危险性防范说明:P305+P351+P338
-
危险性描述:H319
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: N-Acetyltyramine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: N-Acetyltyramine
CAS number: 1202-66-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H13NO2
Molecular weight: 179.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: N-Acetyltyramine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: N-Acetyltyramine
CAS number: 1202-66-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H13NO2
Molecular weight: 179.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对羟基苯乙胺 tyrosamine 51-67-2 C8H11NO 137.181 4-(2-乙酰氨基基乙基)苯基乙酸酯 Diacetyl-tyramin 14383-56-3 C12H15NO3 221.256 N-Boc-酪胺 N-t-butoxycarbonyl-tyramine 64318-28-1 C13H19NO3 237.299 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-[2-(4-甲氧基苯基)乙基]乙酰胺 N-(4-methoxyphenylethyl)acetamide 54815-19-9 C11H15NO2 193.246 对羟基苯乙胺 tyrosamine 51-67-2 C8H11NO 137.181 4-(2-乙酰氨基基乙基)苯基乙酸酯 Diacetyl-tyramin 14383-56-3 C12H15NO3 221.256 N-[2-(4-氨基苯基)乙基]乙酰胺 4-(2-(((methyl)carbonyl)amino)ethyl)aniline 40377-41-1 C10H14N2O 178.234 —— 4-<2-(Acetamido)-ethyl>-phenoxyessigsaeureethylester 55458-52-1 C14H19NO4 265.309 —— N-[2-(3,5-dichloro-4-hydroxyphenyl)ethyl]acetamide 437712-24-8 C10H11Cl2NO2 248.109 —— N-(4-hydroxy-3,5-diiodo-phenethyl)-acetamide 90468-81-8 C10H11I2NO2 431.012 —— N-acetyl-3,5-dibromo-4-hydroxylphenylethamine 99070-34-5 C10H11Br2NO2 337.011
反应信息
-
作为反应物:参考文献:名称:水稻组蛋白脱乙酰基酶10和拟南芥组蛋白脱乙酰基酶14基因编码N-乙酰5-羟色胺脱乙酰酶,催化N-乙酰5-羟色胺转化为5-羟色胺,这是植物褪黑素生物合成的逆反应。摘要:在植物中,褪黑激素的产生受到严格的调节,这与其褪色素的前体的产生不同,后者是对衰老和病原体暴露等刺激产生高度诱导作用的。外源性5-羟色胺处理不会在植物中极大地诱导N-乙酰5-羟色胺(NAS)和褪黑激素的产生,这表明从5-羟色胺生物合成褪黑激素的途径中可能存在一个或多个调控基因。在此报告中,我们发现NAS在水稻幼苗中迅速大量转化为5-羟色胺,表明存在N-乙酰5-羟色胺脱乙酰基酶(ASDAC)。为了克隆假定的ASDAC基因,我们筛选了4个被称为组蛋白脱乙酰基酶(HDAC)的基因。)基因,但编码的蛋白质靶向叶绿体或线粒体而不是细胞核。在表达这些基因的4种重组大肠杆菌菌株中,发现一种表达水稻HDAC10基因的大肠杆菌菌株能够响应于NAS处理而产生5-羟色胺。重组纯化的水稻HDAC10(OsHDAC10)蛋白对NAS,N-乙酰酪胺(NAT),N具有ASDAC酶活性-乙酰色胺和褪黑激素,对于NAT具有DOI:10.1111/jpi.12460
-
作为产物:参考文献:名称:Phenoxyalkylcarboxylic acid compounds and thrombocyte-aggregation摘要:该公式中的Phenoxyalkylcarboxylic酸化合物,其中R是氢或较低的烷基,R.sub.1是烷基或芳基,芳基可以被卤素、羟基、三氟甲基或较低的烷基、烷氧基或酰基取代一次或多次,R.sub.2和R.sub.3分别选自氢或较低的烷基,n为0、1、2或3,其生理上可接受的盐、酯和酰胺在抑制血小板聚集方面具有出色的效果。公开号:US04258058A1
文献信息
-
Characterization of Arylalkylamine <i>N</i>-Acyltransferase from <i>Tribolium castaneum</i>: An Investigation into a Potential Next-Generation Insecticide Target作者:Brian G. O’Flynn、Eric M. Lewandowski、Karin Claire Prins、Gabriela Suarez、Angelica N. McCaskey、Nasha M. Rios-Guzman、Ryan L. Anderson、Britney A. Shepherd、Ioannis Gelis、James W. Leahy、Yu Chen、David J. MerklerDOI:10.1021/acschembio.9b00973日期:2020.2.21short-chain acyl-CoAs (C2-C10), benzoyl-CoA, and succinyl-CoA functioning in the role of acyl donor. Recombinant TcAANAT0 was expressed and purified from E. coli and was used to investigate the kinetic and chemical mechanism of catalysis. The kinetic mechanism is an ordered sequential mechanism with the acyl-CoA binding first. pH-rate profiles and site-directed mutagenesis studies identified amino acids critical杀虫剂抗性问题日益严重,这意味着确定新的杀虫剂目标变得前所未有的重要。芳烷基胺 N-酰基转移酶 (AANATs) 已被建议作为潜在的新目标。这些混杂的酶参与生物胺的 N-酰化以形成 N-酰胺。在昆虫中,这个过程是黑色素、角质层硬化、生物胺去除和脂肪酸酰胺生物合成的关键步骤。表征的每个 AANAT 同种型的独特性质表明每个生物体都容纳了该生物体相对专有的离散 AANAT 组装。这意味着在杀虫剂设计中具有很高的选择性,同时也保持了多药性。此处介绍了对 AANAT 的全面动力学和结构分析,该分析在世界上所有植物商品中最常见的次生害虫之一 Tribolium castaneum 中发现。这种名为 TcAANAT0 的酶催化短链 N-酰基芳基烷基胺的形成,其中短链酰基辅酶 A (C2-C10)、苯甲酰辅酶 A 和琥珀酰辅酶 A 在酰基供体的作用下起作用。从大肠杆菌中表达和纯化重组 TcAANAT0,
-
Microwave‐Assisted Deacylation of Unactivated Amides Using Ammonium‐Salt‐Accelerated Transamidation作者:Yuhei Shimizu、Hiroyuki Morimoto、Ming Zhang、Takashi OhshimaDOI:10.1002/anie.201202354日期:2012.8.20The combination of an ammonium salt and ethylenediamine promotes deacylation of a variety of unactivated amides to give the corresponding amines in high yields without the use of strong acids or bases. The reactions proceed without special care regarding air and moisture, and tolerate a wide range of functional groups.
-
An Unconventional Reaction of 2,2-Diazido Acylacetates with Amines作者:Andreas P. Häring、Phillip Biallas、Stefan F. KirschDOI:10.1002/ejoc.201601625日期:2017.3.17discovered that 2,2-diazido acylacetates, a class of compounds with essentially unknown reactivity, can be coupled to amines through a new strategy that does not involve any reagents. 2,2-Diazido acetate is the unconventional leaving group under carbon–carbon bond cleavage. This reaction leads to the construction of amide bonds, tolerates various functionalities and is performed equally well in numerous
-
A USEFUL METHOD FOR SELECTIVE ACYLATION OF ALCOHOLS USING 2,2′-BIPYRIDYL-6-YL CARBOXYLATE AND CESIUM FLUORIDE作者:Teruaki Mukaiyama、Fong-Chang Pai、Makoto Onaka、Koichi NarasakaDOI:10.1246/cl.1980.563日期:1980.5.5Primary and secondary alcohols are acylated under mild conditions by the use of 2,2′-bipyridyl-6-yl carboxylates and cesium fluoride. Furthermore, the reaction is successfully applied to selective acylation of a primary carbinol group of diols containing primary and secondary carbinol groups or exclusive O-acylation of aromatic amino alcohols.
-
Pyrimidine derivatives and processes for the preparation thereof申请人:Yuhan Corporation公开号:US06352993B1公开(公告)日:2002-03-05The present invention relates to novel pyrimidine derivatives of formula (I) or pharmaceutically acceptable salts thereof which possess an excellent anti-secretory activity, pharmaceutical compositions containing the same as an active ingredient, their novel intermediates, and processes for the preparation thereof wherein: when A is piperidin-1-yl or —NH—B, wherein B is C3-C4 alkyl, C3-C4 alkenyl, C3-C7 cycloalkyl, C1-C3 alkoxyethyl, phenylethl which may be substituted or unsubstituted, 3-trifluoromethylphenylmethyl, 1-naphthylmethyl, 4-methylthiazol-2-yl or 4-phenylthiazol-2-yl, R1 is hydrogen or methyl; and R2, R3, R4 and R5 are hydrogen; or when A is a group of formula (II); when R1 is hydroxymethyl or C1-C3 alkoxymethyl, R2, R3, R4, R5 and R6 are hydrogen; and R7 is hydrogen or halogen; or when R1 is hydrogen or methyl, R7 is hydrogen or halogen; and one or two of R2, R3, R4, R5 and R6 is hydroxy, methoxy, or a group of formula (III) wherein Z is C1-C4 alkyl, substituted or unsubstituted C1-C4 alkenyl, cyloalkyl, benzyloxyalkyl, alkoxycarbonylalkyl, morpholinomethyl, piperidinomethyl, 4-substituted-piperazinomethyl, substituted or unsubstituted phenyl, naphthyl, substituted or unsubstituted benzyl, thiophen-2-yl-methyl, 1-substituted-pyrrolidin-2-yl or —CHR8NHR9, wherein R8 is hydrogen, methyl, isopropyl, benzyl, benzyloxymethyl, methylthioethyl, benzyloxycarbonylmethyl, carbamolymethyl, carbamoylethyl, or 1-benzylimidazol-4-ylmethyl and R9 is hydrogen or t-butoxycarbonyl; and the others are hydrogen or methyl.本发明涉及具有优异抗分泌活性的新型嘧啶衍生物的化学式(I)或其药学上可接受的盐,以及含有其作为活性成分的药物组合物,其新型中间体,以及其制备方法,其中:当A为哌啶-1-基或-NH-B时,其中B为C3-C4烷基,C3-C4烯基,C3-C7环烷基,C1-C3烷氧乙基,苯乙基,可以是取代或未取代的,3-三氟甲基苯甲基,1-萘甲基,4-甲基噻唑-2-基或4-苯基噻唑-2-基,R1为氢或甲基;而R2、R3、R4和R5为氢;或当A为化学式(II)的基团时;当R1为羟甲基或C1-C3烷氧甲基,R2、R3、R4、R5和R6为氢;而R7为氢或卤素;或当R1为氢或甲基,R7为氢或卤素;且R2、R3、R4、R5和R6中的一个或两个为羟基,甲氧基,或化学式(III)的基团,其中Z为C1-C4烷基,取代或未取代的C1-C4烯基,环烷基,苄氧基烷基,烷氧羰基烷基,吗啉甲基,哌啶甲基,4-取代哌嗪甲基,取代或未取代的苯基,萘基,取代或未取代的苄基,噻吩-2-基-甲基,1-取代吡咯啉-2-基或-NHR9,其中R8为氢,甲基,异丙基,苄基,苄氧甲基,甲硫乙基,苄氧羰基甲基,氨基甲基,氨基乙基,或1-苄基咪唑-4-基甲基,而R9为氢或叔丁氧羰基;其他为氢或甲基。
表征谱图
-
氢谱1HNMR
-
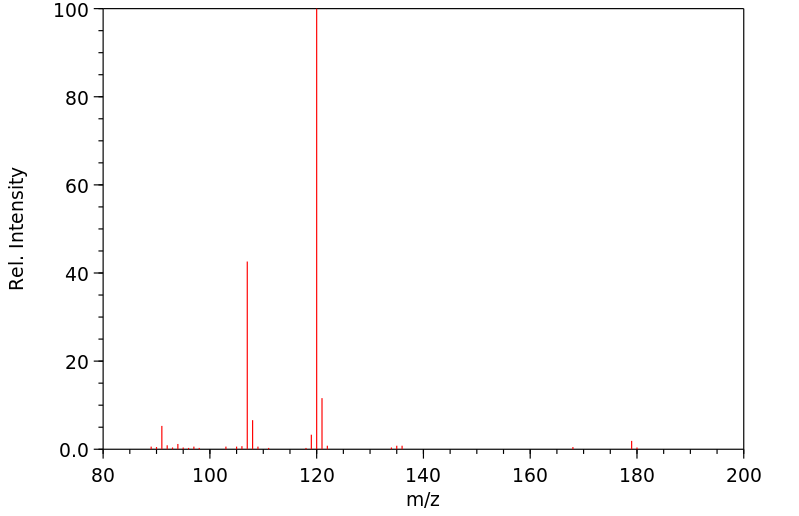
质谱MS
-
碳谱13CNMR
-
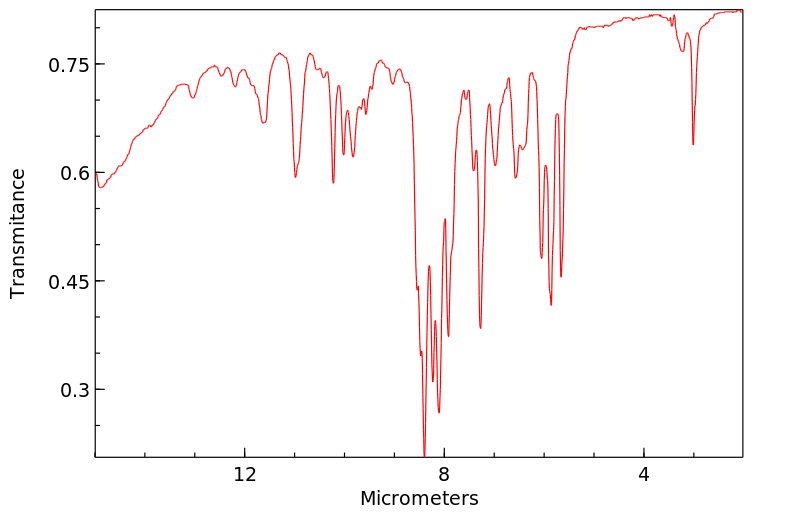
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚