6-甲基-2-庚炔 | 51065-64-6
中文名称
6-甲基-2-庚炔
中文别名
2-庚炔-6-甲基
英文名称
6-methyl-2-heptyne
英文别名
6-Methyl-2-heptin;6-methylhept-2-yne
CAS
51065-64-6
化学式
C8H14
mdl
MFCD00041619
分子量
110.199
InChiKey
HIEALULIKYDRQN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-86.55°C (estimate)
-
沸点:125 °C
-
密度:0,76 g/cm3
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
安全说明:S23,S33,S62
-
危险类别码:R10,R65
-
海关编码:2901299090
-
包装等级:II
-
危险类别:3
-
危险品运输编号:3295
-
储存条件:请将贮藏器密封保存,并将其存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-辛烯-3-炔 oct-1-en-3-yne 17679-92-4 C8H12 108.183
反应信息
-
作为反应物:参考文献:名称:Ondruschka, Bernd; Remmler, Matthias; Zimmermann, Gerhard, Zeitschrift fur Chemie, 1988, vol. 28, # 8, p. 307 - 308摘要:DOI:
-
作为产物:描述:参考文献:名称:Alkylidenecarbenes from acyclic vinyl bromides and potassium tert-butoxide摘要:DOI:10.1021/jo00867a001
文献信息
-
Fluoroselenenylation of Alkynes作者:Yoshinosuke Usuki、Michio Iwaoka、Shuji TomodaDOI:10.1246/cl.1992.1507日期:1992.8Benzeneselenenyl fluoride equivalent was generated in situ by the reaction of silver(I) fluoride with benzeneselenenyl bromide in dichloromethane under ultrasound irradiation. Treatment of internal alkynes with this reagent afforded 2-fluoro-1-alkenyl phenyl selenides in moderate yields.
-
Iridium Catalyzed Carbocyclizations: Efficient (5+2) Cycloadditions of Vinylcyclopropanes and Alkynes作者:Michaela-Christina Melcher、Henrik von Wachenfeldt、Anders Sundin、Daniel StrandDOI:10.1002/chem.201405729日期:2015.1.7Third‐row transition metal catalysts remain a largely untapped resource in cycloaddition reactions for the formation of medium‐sized rings. Herein, we report the first examples of iridium‐catalyzed inter‐ and intramolecular vinylcyclopropane (VCP)–alkyne (5+2) cycloadditions. DFT modeling suggests that catalysis by iridium(I) proceeds through a mechanism similar to that previously reported for rhodium(I)‐catalyzed
-
Rhodium(III)-Catalyzed Arene and Alkene C−H Bond Functionalization Leading to Indoles and Pyrroles作者:David R. Stuart、Pamela Alsabeh、Michelle Kuhn、Keith FagnouDOI:10.1021/ja1082624日期:2010.12.29formation of indoles via the oxidative annulation of acetanilides with internal alkynes. The optimized reaction conditions allow for molecular oxygen to be used as the terminal oxidant in this process, and the reaction may be carried out under mild temperatures (60 °C). These conditions have resulted in an expanded compatibility of the reaction to include a range of new internal alkynes bearing synthetically最近,铑 (III) 复合物 [Cp*RhCl(2)](2) 1 为基于铑催化的 CH 键功能化事件的芳香杂环的有效合成提供了令人兴奋的机会。在本报告中,配合物 1 及其双阳离子类似物 [Cp*Rh(MeCN)(3)][SbF(6)](2) 2 的使用已用于通过乙酰苯胺与内部炔烃。优化的反应条件允许分子氧在该过程中用作末端氧化剂,并且反应可以在温和的温度(60°C)下进行。这些条件导致反应的相容性扩大,包括一系列带有合成有用官能团的新内部炔烃,产率中等至极好。该方法的适用性在 paullone 3 的有效合成中得到了例证,它是一种具有既定生物活性的四环吲哚衍生物。使用氘标记实验和动力学分析对反应进行的机理研究提供了对偶联伙伴反应性问题的深入了解,并有助于开发提高间位取代乙酰苯胺的区域选择性的条件。该反应类别也已扩展到包括吡咯的合成。催化剂 2 在室温下有效地将取代的烯酰胺与内部炔烃偶联以形成三取代的吡咯,收率良好至极好。
-
A highly selective cobalt-catalyzed carbonylative cyclization of internal alkynes with carbon monoxide and organic thiols作者:Yoshihiro Higuchi、Shinya Higashimae、Taichi Tamai、Akiya OgawaDOI:10.1016/j.tet.2013.10.080日期:2013.12ineffective, cobalt carbonyl (Co2(CO)8) is an excellent catalyst for carbonylative cyclization of internal alkynes with carbon monoxide. When Co2(CO)8-catalyzed reactions of internal alkynes with organic thiols are conducted in acetonitrile under 4 MPa pressure of carbon monoxide, thiolative lactonization of internal alkynes successfully takes place with incorporation of two molecules of CO. This carbonylation
-
An Efficient and Highly Selective Carbonylative Bisthiolation of Internal Alkynes with Organic Disulfides Catalyzed by [Co<sub>2</sub>(CO)<sub>8</sub>]作者:Yoshihiro Higuchi、Shinya Higashimae、Taichi Tamai、Akihiro Nomoto、Motohiro Sonoda、Akiya OgawaDOI:10.1246/cl.130441日期:2013.10.5Although many transition-metal catalysts are ineffective for the addition and carbonylative addition of organic disulfides to internal alkynes, dicobalt octacarbonyl ([Co2(CO)8]) was found to exhib...
表征谱图
-
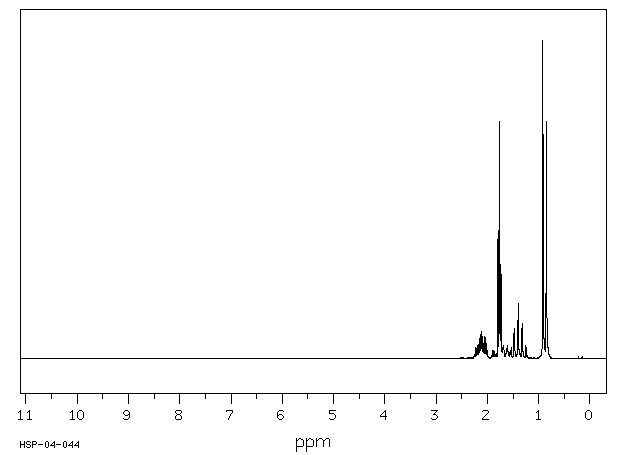
氢谱1HNMR
-
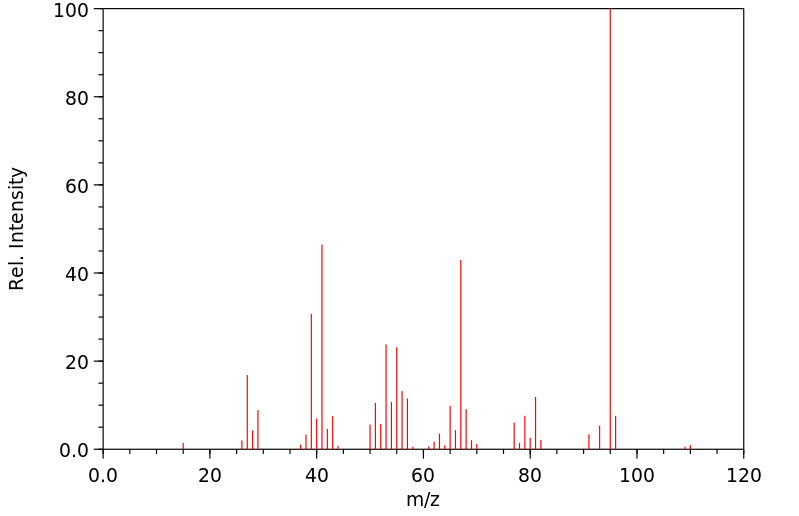
质谱MS
-
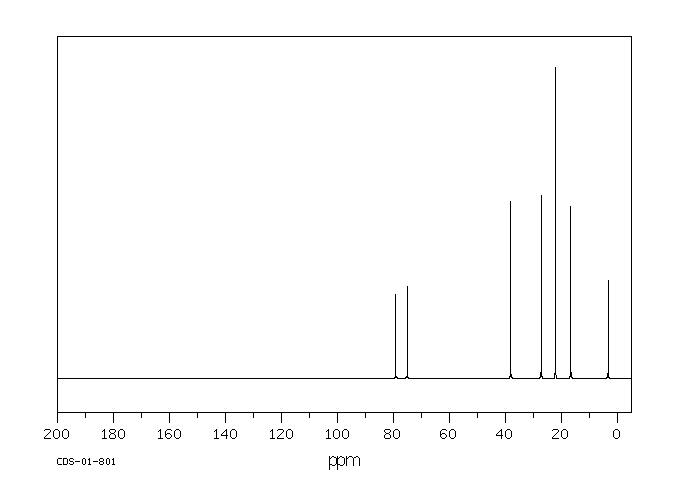
碳谱13CNMR
-
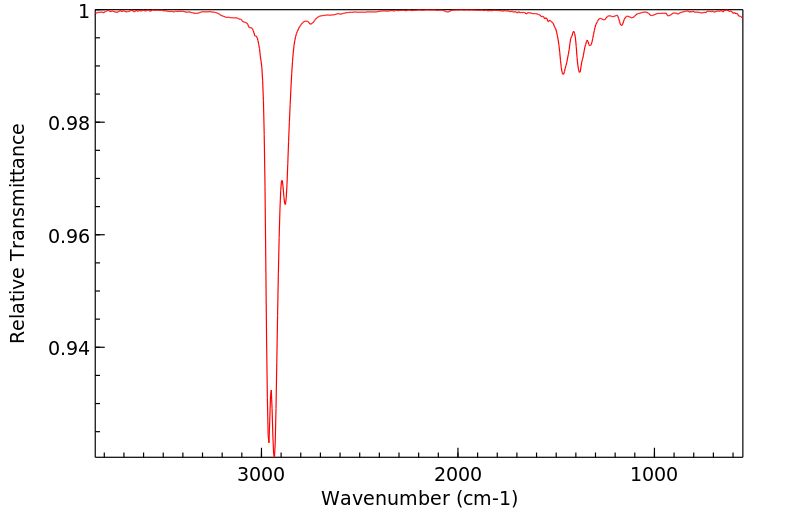
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-