cis-1,2-dihydrocatechol | 17793-95-2
中文名称
——
中文别名
——
英文名称
cis-1,2-dihydrocatechol
英文别名
cis-3,5-cyclohexadiene-1,2-diol;(+)-cis-2,3-dihydroxy-cyclohexa-4,6-diene;cis-Cyclohexa-3,5-diene-1,2-diol;(1S,2R)-cyclohexa-3,5-diene-1,2-diol
CAS
17793-95-2
化学式
C6H8O2
mdl
——
分子量
112.128
InChiKey
YDRSQRPHLBEPTP-OLQVQODUSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:60 °C(Solv: hexane (110-54-3))
-
沸点:226.4±28.0 °C(Predicted)
-
密度:0.904 g/mL at 25 °C
-
闪点:-3 °C
计算性质
-
辛醇/水分配系数(LogP):-0.1
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险品标志:F,Xi
-
危险类别码:R36/37/38,R11
-
危险品运输编号:UN 1173 3/PG 2
-
海关编码:2906199090
-
安全说明:S16,S23,S26,S29,S33
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— cis-5,6-dimethoxy-1,3-cyclohexadiene 110851-49-5 C8H12O2 140.182
反应信息
-
作为反应物:参考文献:名称:合成中的微生物氧化:由苯制备(+)-和(-)-松醇摘要:用恶臭假单胞菌(Pseudomonas putida)对苯进行微生物氧化可得到顺式-1,2-二羟基环己-3,5-二烯(2),可以分五个步骤进行转化,总产率为49%,为(±)-松醇。用薄荷醇乙酰氯拆分中间体醇(6)可提供光学纯的物质,可将其独立转化为(+)-或(-)-松醇。研究了松醇的脱甲基条件,以及(2)和相关化合物的进一步反应。DOI:10.1016/s0040-4020(01)81025-5
-
作为产物:描述:α-3,4,5,6-Tetrachlorocyclohex-1-ene 在 potassium permanganate 、 乙醇 、 magnesium sulfate 、 锌 作用下, 生成 cis-1,2-dihydrocatechol参考文献:名称:Nakajima et al., Chemische Berichte, 1959, vol. 92, p. 163,166,170摘要:DOI:
文献信息
-
Gas-phase reaction of OH radicals with benzene: products and mechanism作者:Torsten Berndt、Olaf BögeDOI:10.1039/b106667f日期:——The gas-phase reaction of OH radicals with benzene was studied in O2/He mixtures under flow conditions in the temperature range 276–353 K and at pressures of 100 and 500 mbar using on-line FT-IR spectroscopy and GC-MS measurements. The reaction conditions were chosen so that the initially formed OH/benzene adduct predominantly reacted either with O2 or O3. Under conditions of a predominant reaction使用在线 FT-IR 光谱和 GC-MS 测量,在 276–353 K 温度范围和 100 和 500 mbar 压力下,在 O2/He 混合物中研究了 OH 自由基与苯的气相反应。选择反应条件以使最初形成的OH/苯加合物主要与O2或O3反应。在 OH/苯加合物与 O2 的主要反应条件下,研究了不同 NO 浓度的产物形成。鉴定产物为六-2,4-二烯二醛、苯酚、硝基苯、对苯醌和乙二醛的异构体。发现少量呋喃。随着 NO 浓度的增加,苯酚产率降低,反式、反式-六-2,4-二烯二烯和硝基苯的产率增加,最大值分别为 0.36 ± 0.02 和 0.11 ± 0.02,分别(100 毫巴,295 K)。发现 0.08 ± 0.02 的对苯醌产率与 NO 浓度无关。在 500 mbar 下,[NO]/[O2] = 1-20 × 10-6 的初始比率在 276-353 K 范围内测量苯酚产率的温度依赖性。对于固定的
-
Biocatalytic Asymmetric Dihydroxylation of Conjugated Mono- and Poly-alkenes to Yield Enantiopure Cycliccis-Diols作者:Derek R. Boyd、Narain D. Sharma、Nigel I. Bowers、Ian N. Brannigan、Melanie R. Groocock、John F. Malone、Gareth McConville、Christopher C. R. AllenDOI:10.1002/adsc.200505033日期:2005.6at benzylic or allylic centres. Competition from allylic hydroxylation of methyl groups was observed only when naphthalene dioxygenase was used as biocatalyst. The structures, enantiomeric excess values and absolute configurations of the bioproducts, were determined by a combination of stereochemical correlation, spectroscopy (NMR and CD) and X-ray diffraction methods. cis-1,2-Diol metabolites from发现一系列共轭单烯烃和多烯的双加氧酶催化的不对称二羟基化反应产生相应的单醇和1,2-二氢二醇。从单取代得到的二醇的代谢物,宝石二取代的,顺式二取代的,三取代的和烯烃底物,使用的全细胞恶臭假单胞菌含有甲苯和萘双加氧酶的菌株。确定双加氧酶的选择和烯烃类型是重要因素,优选双加氧酶催化的共轭烯烃或芳烃基团的1,2-二羟基化,以及苄基或烯丙基中心的单羟基化。仅当使用萘二加氧酶作为生物催化剂时,才能观察到甲基的烯丙基羟化竞争。生物产物的结构,对映体过量值和绝对构型是通过立体化学相关性,光谱学(NMR和CD)和X射线衍射方法的组合来确定的。顺式通常观察到来自芳烃,环状烯烃和二烯的-1,2-二醇代谢物是对映纯的(> 98%ee),而来自无环烯烃的1,2-二醇的对映体过量值较低(<88%ee)。宝石-二取代的富勒烯的对映体纯的顺式-二醇代谢物被用作新的化学酶促途径中的前驱体,以生成新型的C 2-对称的酮。
-
Chemistry of <i>anti-o,o</i>‘-Dibenzene作者:Taehee Noh、Hong Gan、Sharon Halfon、Bruce J. Hrnjez、Nien-chu C. YangDOI:10.1021/ja970199o日期:1997.8.1anti-tetraol 23 was converted to 1 in 65% yield on a 0.5 g scale. This has allowed us to explore the chemistry of anti-dibenzenes extensively. The kinetics for thermal reversion of 1 to benzene have been studied in three different solvents. The direct photolysis of 1 to benzene has been found to form excited benzene in unit efficiency. This high efficiency of adiabatic photon up-conversion in the singlet manifold
-
Catalytic behaviour of chloroperoxidase from Caldariomyces fumago in the oxidation of cyclic conjugated dienes作者:Claudia Sanfilippo、Giovanni NicolosiDOI:10.1016/s0957-4166(02)00509-8日期:2002.9Chloroperoxidase from Caldariomyces fumago has been investigated as a catalyst for the oxidation of cyclic conjugated dienes. The nature of the substituents and the size of the carbocycle affect the enantioselectivity of the enzyme. An unexpected course of the CPO-catalyzed oxidation has been observed in the reaction of cis,cis-1,3-cyclooctadiene.
-
Chemoenzymatic synthesis of monocyclic arene oxides and arene hydrates from substituted benzene substrates作者:Derek R. Boyd、Narain D. Sharma、Vera Ljubez、Peter K. M. McGeehin、Paul J. Stevenson、Marine Blain、Christopher C. R. AllenDOI:10.1039/c3ob40166a日期:——Reduction of a substituted arene oxide to yield a racemic arene hydrate was observed. Arene hydrates have also been synthesised, in enantiopure form, from the corresponding dihydroarene oxide or trans-bromoacetate precursors. Biotransformation of one arene hydrate enantiomer resulted in a toluene-dioxygenase catalysed cis-dihydroxylation to yield a benzene cis-triol metabolite.
表征谱图
-
氢谱1HNMR
-
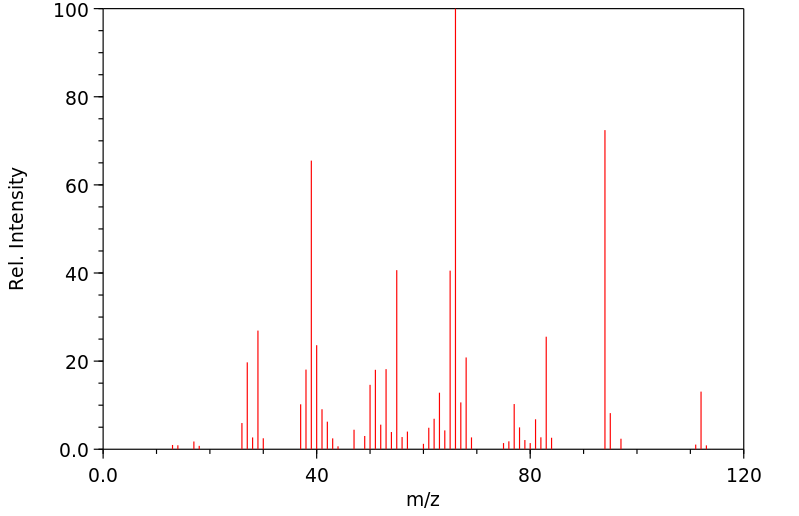
质谱MS
-
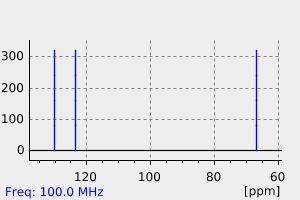
碳谱13CNMR
-
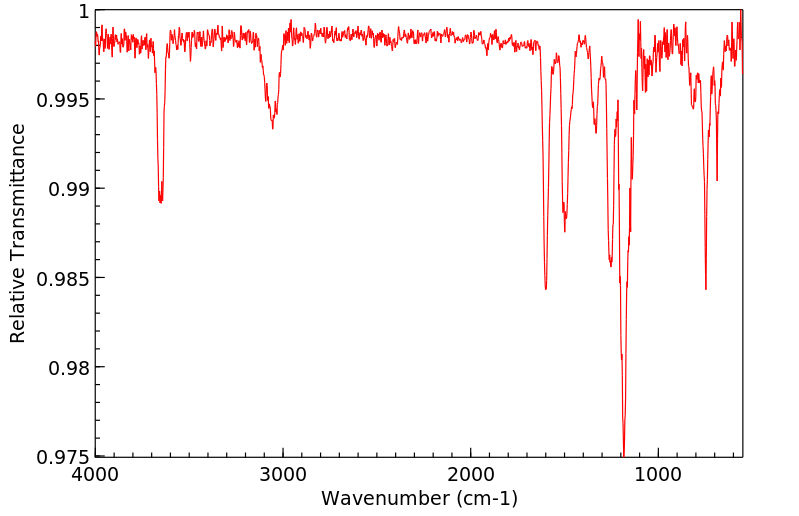
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷