2-nonene | 2216-38-8
中文名称
——
中文别名
——
英文名称
2-nonene
英文别名
non-2-ene
CAS
2216-38-8
化学式
C9H18
mdl
——
分子量
126.242
InChiKey
IICQZTQZQSBHBY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-89.14°C (estimate)
-
沸点:144-145 °C(lit.)
-
密度:0.734 g/mL at 25 °C(lit.)
-
闪点:90 °F
-
物理描述:Nonene appears as a clear colorless liquid with a sharp odor. Flash point 75°F. Insoluble in water and less dense than water. Hence floats on water. May irritate skin on contact. Inhalation of vapors may cause irritation. Prolonged inhalation may lead to breathing difficulty. Ingestion causes abdominal discomfort, nausea and diarrhea.
-
颜色/状态:COLORLESS LIQUID
-
蒸汽压力:Vapor pressure = 3.75 mm Hg @ 25 °C
-
粘度:0.851 sq mm/s @ 20 °C
-
燃烧热:-10,600 CAL/G
-
汽化热:68.9 CAL/G (EST)
-
表面张力:22 DYNES/CM
-
保留指数:909;902
计算性质
-
辛醇/水分配系数(LogP):4.8
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.78
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
安全说明:S16,S29,S33
-
危险类别码:R10
-
海关编码:2901299090
-
危险品运输编号:UN 3295 3/PG 3
-
储存条件:请将容器密封保存,并存放在干燥、阴凉的地方。
SDS
2-壬烯 (顺反异构体混合物)
模块 1. 化学品
产品名称: 2-Nonene (cis- and trans- mixture)
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-壬烯 (顺反异构体混合物)
百分比: >94.0%(GC)
CAS编码: 2216-38-8
2-壬烯 (顺反异构体混合物)
模块 3. 成分/组成信息
分子式: C9H18
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于冷藏防爆冰箱。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
热敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
2-壬烯 (顺反异构体混合物)
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.74
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
2-壬烯 (顺反异构体混合物)
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
《危险化学品名录(2002版)》CN编号:33514
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Nonene (cis- and trans- mixture)
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-壬烯 (顺反异构体混合物)
百分比: >94.0%(GC)
CAS编码: 2216-38-8
2-壬烯 (顺反异构体混合物)
模块 3. 成分/组成信息
分子式: C9H18
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于冷藏防爆冰箱。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
热敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
2-壬烯 (顺反异构体混合物)
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.74
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
2-壬烯 (顺反异构体混合物)
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
《危险化学品名录(2002版)》CN编号:33514
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:钯催化的分子氧对烯烃的直接氧化:制备1,2-二元醇,醛和酮的通用和实用方法摘要:1,2-二醇,醛和酮是化学合成中的重要中间体,烯烃是1,2-二醇,醛和酮的可能前体。在此,提出了新颖的且环境友好的方法,该方法用于钯催化的二羟基化作用以及用氧作为唯一氧化剂的烯烃的氧化裂解。裂解反应是用酸作为添加剂在水溶液中进行的,而1,2-二醇是在碱的存在下形成的。已经证明广泛的底物范围允许单取代的芳族和脂族末端烯烃,1,2-二取代的和1,1-二取代的烯烃。二氧-Pd II配合物的裂解反应意味着1,2-二醇可能是烯烃裂解的关键中间体。DOI:10.1021/jo100125q
-
作为产物:参考文献:名称:[EN] PRODUCING ALPHA-OLEFINS
[FR] PRODUCTION D'ALPHA-OLÉFINES摘要:生产α-烯烃的方法。该方法包括选择性异构化α-烯烃以形成β-烯烃和将至少一部分β-烯烃混合物乙烯化为α-烯烃。公开号:WO2011081650A1
文献信息
-
Ionic Liquid Stabilized Niobium Oxoclusters Catalyzing Oxidation of Sulfides with Exceptional Activity作者:Qingqing Zhou、Man Ye、Wenbao Ma、Difan Li、Bingjie Ding、Manyu Chen、Yefeng Yao、Xueqing Gong、Zhenshan HouDOI:10.1002/chem.201806178日期:2019.3.15underwent structural transformation in the presence of H2O2 but regenerated to their initial state at the end of the reaction. In particular, the highly dispersed Nb oxoclusters can absorb a large amount of polar organic solvents and thus were swollen greatly, which exhibited “pseudo” liquid phase behavior, and enabled the substrate molecules to be highly accessible to the catalytic center of Nb oxocluster我们在这里提出了一种新型的铌氧簇,它可以通过羧酸盐离子液体有效地稳定下来。这些功能化的IL在本工作中分别命名为[TBA] [LA],[TBA] [PA]和[TBA] [HPA],其中TBA代表四丁基铵,LA,PA和HPA分别指乳酸,丙酸酯,3 -羟基丙酸酯阴离子。已通过元素分析,NMR,IR,XRD,TGA和HRTEM对合成的Nb氧簇进行了表征。发现[TBA] [LA]稳定的Nb氧簇(Nb-OC @ [TBA] [LA])均匀分散,平均粒径为2-3 nm,并具有极高的催化活性,可选择性氧化各种硫醚。在催化剂负载量低至0.0033 mol%(1 ppm)时,Nb-OC @ [TBA] [LA]催化剂的周转数超过56000。与此同时,通过仅使用0.065mol%的催化剂(50ppm),该催化剂还显示出对烯烃和烯丙基醇的环氧化的高活性。的表征93 Nb NMR光谱表明,Nb氧簇在H 2 O 2存
-
Highly selective and efficient olefin epoxidation with pure inorganic-ligand supported iron catalysts作者:Zhuohong Zhou、Guoyong Dai、Shi Ru、Han Yu、Yongge WeiDOI:10.1039/c9dt02997d日期:——efficient and selective epoxidation of alkenes via the design of specialized ligands, which facilitates to control the activity and selectivity of the reactions catalyzed by iron atom. Herein, we report the development of the olefin epoxidation with inorganic-ligand supported iron-catalysts using 30% H2O2 as an oxidant, and the mechanism is similar to iron-porphyrin type. With the catalyst 1, (NH4)3[FeMo6O18(OH)6]在过去的二十年中,在过渡铁催化的烯烃选择性氧化为环氧化物方面取得了重大进展。在药物,分离的天然产物和精细化学品中发现的常见结构。这些方法中的许多已通过专门配体的设计实现了烯烃的高效和选择性环氧化,这有助于控制铁原子催化的反应的活性和选择性。本文中,我们报道了使用30%H 2 O 2作为氧化剂的无机配体负载的铁催化剂进行烯烃环氧化的研究进展,其机理与铁卟啉类型相似。对于催化剂1,(NH 4)3 [FeMo 6O 18(OH)6 ],各种芳族和脂肪族烯烃均以优异的收率以及化学和立体选择性成功地转化为相应的环氧化物。该催化体系具有能够避免使用昂贵的,有毒的,对空气/湿气敏感的和商业上不可用的有机配体的优点。该方法的通用性易于操作,具有较高的催化活性和优异的稳定性,这使其有可能在工业规模上使用,并可能为通过无机配体配位的铁催化进行催化氧化反应开辟一条道路。。
-
Metal-Free Catalytic Reductive Cleavage of Enol Ethers作者:Karina Chulsky、Roman DobrovetskyDOI:10.1021/acs.orglett.8b02932日期:2018.11.2In contrast to the well-known reductive cleavage of the alkyl–O bond, the cleavage of the alkenyl–O bond is much more challenging especially using metal-free approaches. Unexpectedly, alkenyl–O bonds were reductively cleaved when enol ethers were reacted with Et3SiH and a catalytic amount of B(C6F5)3. Supposedly, this reaction is the result of a B(C6F5)3-catalyzed tandem hydrosilylation reaction and
-
Methylformate as replacement of syngas in one-pot catalytic synthesis of amines from olefins作者:Eduard Karakhanov、Anton Maksimov、Yulia Kardasheva、Elena Runova、Roman Zakharov、Maria Terenina、Corey Kenneally、Victor ArredondoDOI:10.1039/c3cy00862b日期:——general approach for the one-pot hydroaminomethylation of olefins using methylformate as formylating agent instead of synthesis gas (syngas) has been proposed. Herein we report that a Ru–Rh catalytic system demonstrates high activity in a tandem conversion of a series of n-alkenes into amines using methylformate with yields 58–92% (6 h). The selectivity for the normal amine reached 96% with catalysis by
-
Hydroformylation in perfluorinated solvents; improved selectivity, catalyst retention and product separation作者:Douglas F Foster、David Gudmunsen、Dave J Adams、Alison M Stuart、Eric G Hope、David J Cole-Hamilton、Gary P Schwarz、Peter PogorzelecDOI:10.1016/s0040-4020(02)00215-6日期:2002.5hydroformylation of linear terminal alkenes using rhodium based catalysts under fluorous biphasic conditions in the presence and absence of toluene is reported. Using fluorinated ponytails to modify triarylphosphites and triarylphosphines, good selectivities and reactivities can be obtained, along with good retention of the catalyst and ligand within the fluorous phase. Using P(O–4-C6H4C6F13)3 (P/Rh=3:1) as the据报道,在氟的双相条件下,在存在和不存在甲苯的情况下,使用铑基催化剂将线性末端烯烃加氢甲酰化。使用氟化的马尾辫来修饰亚磷酸三芳基酯和亚磷酸三芳基酯,可以获得很好的选择性和反应性,以及催化剂和配体在氟相中的良好保留。使用P(O–4-C 6 H 4 C 6 F 13)3(P / Rh = 3∶1)作为甲苯/全氟-1,3-二甲基环己烷中的配体,在60℃下获得了良好的结果,但是随着温度的升高,催化剂和/或配体发生分解。通过以更高的速率,更好的l / b比以及以全氟化碳溶剂形式更好地保留催化剂和亚磷酸酯而省略甲苯,可以获得更令人印象深刻的结果。竞争性异构化将线性醛的选择性限制在<76%。当P(4-C 6 H 4 C 6 F 13)3在没有甲苯的情况下将其用作配体,甚至可以获得更令人印象深刻的结果,线性醛的选择性高达80.9%,高速率,保留率高达99.95%的铑和高达96.7%的膦在氟相中。将这些结果
表征谱图
-
氢谱1HNMR
-
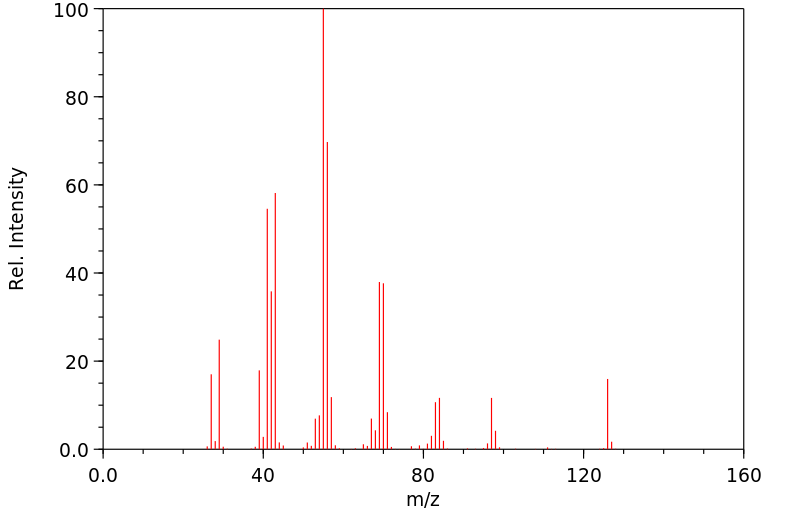
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-