ethyl 2-oxo-1,2-dihydroquinoline-4-carboxylate
中文名称
——
中文别名
——
英文名称
ethyl 2-oxo-1,2-dihydroquinoline-4-carboxylate
英文别名
ethyl 2-hydroxyquinoline-4-carboxylate
CAS
——
化学式
C12H11NO3
mdl
MFCD01925526
分子量
217.224
InChiKey
WTHATVQLTWYSCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:55.4
-
氢给体数:1
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙氧基喹啉-4-羧酸乙酯 2-ethoxy-quinoline-4-carboxylic acid ethyl ester 5471-39-6 C14H15NO3 245.278 2-羟基喹啉-4-羧酸 2-quinolone-4-carboxylic acid 15733-89-8 C10H7NO3 189.17 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6-amino-2-hydroxy-quinoline-4-carboxylic acid ethyl ester 100143-02-0 C12H12N2O3 232.239 1-甲基-2-氧代-1,2-二氢喹啉-4-羧酸乙酯 ethyl 1-methyl-2-oxo-1,2-dihydroquinoline-4-carboxylate 412335-56-9 C13H13NO3 231.251 —— 2-hydroxy-6-nitro-quinoline-4-carboxylic acid ethyl ester 104396-39-6 C12H10N2O5 262.222
反应信息
-
作为反应物:描述:ethyl 2-oxo-1,2-dihydroquinoline-4-carboxylate 在 一水合肼 作用下, 以 乙醇 为溶剂, 以68%的产率得到2-oxo-1,2-dihydroquinoline-4-carbohydrazide参考文献:名称:Construction and functionalization of fused pyridine ring leading to novel compounds as potential antitubercular agents摘要:A series of fused and functionalized pyridine derivatives were designed, synthesized and tested for their potential antitubercular properties. All these novel compounds were prepared by using multistep methods involving the construction of pyridine ring as a key synthetic step. Some of these compounds were found to be interesting when tested for their antitubercular properties in vitro and one of them appeared as an attractive and potential antitubercular agent. (C) 2012 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2012.05.096
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 生成 ethyl 2-oxo-1,2-dihydroquinoline-4-carboxylate参考文献:名称:Koenigs; Koerner, Chemische Berichte, 1883, vol. 16, p. 2158摘要:DOI:
文献信息
-
New alkyl (cyclohexyl) 2-oxo-1-(prop‑2-yn-1-yl)-1, 2-dihydroquinoline-4-carboxylates: Synthesis, crystal structure, spectroscopic characterization, hirshfeld surface analysis, molecular docking studies and DFT calculations作者:Sonia Hayani、Yusuf Sert、Yassir Filali Baba、Fouad Benhiba、Fouad Ouazzani Chahdi、Fatim-Zahra Laraqui、Joel T. Mague、Brahim El Ibrahimi、Nada Kheira Sebbar、Youssef Kandri Rodi、El Mokhtar EssassiDOI:10.1016/j.molstruc.2020.129520日期:2021.3Abstract An efficient synthesis of novel alkyl 2-oxo-1,2-dihydroquinoline-4-carboxylates (3a-3d) was developed, starting from 2-oxo-quinoline carboxylic acid 1a which successively undergoes esterification and alkylation reactions. The structures of all the obtained compounds were investigated and characterized by using 1H NMR and 13C NMR spectroscopic measurements. The molecular and crystal structures摘要 以 2-oxo-quinoline 羧酸 1a 为原料,通过酯化和烷基化反应,高效合成了新型 2-oxo-1,2-dihydroquinoline-4-carboxylates (3a-3d)。通过使用 1H NMR 和 13C NMR 光谱测量研究和表征所有获得的化合物的结构。四种化合物(3a、3b、3c 和 3d)的分子和晶体结构也已通过单晶 X 射线晶体学进行了检查。此外,还使用密度泛函理论 (DFT) 在 B3LYP/6-31G(d,p) 理论水平上获得了实验数据和预测光谱数据。此外,通过 Hirshfeld 表面分析、分子对接研究和 DFT 计算确定了四种结构的活性原子之间的最密切接触。实验结果与计算结果具有相关性,显示出很好的兼容性。最后,进行分子对接研究以研究标题化合物与蛋白质数据库(PDB:1M17-EGFR 激酶)抑制剂靶标的结合模式,并使用 Auto-Dock Vina
-
Pd-Catalyzed Direct Cross-Coupling of Electron-Deficient Polyfluoroarenes with Heteroaromatic Tosylates作者:Shilu Fan、Jie Yang、Xingang ZhangDOI:10.1021/ol201706t日期:2011.8.19We report a Pd-catalyzed direct cross-coupling of electron-deficient polyfluoroarenes with heteroaromatic tosylates. The notable features of this reaction are its high reaction efficiency, excellent chemoselectivity, operational simplicity, and mild reaction conditions. We have applied this protocol to prepare the semiconducting materials in a highly efficient manner.
-
Enantioselective, Intermolecular [<sub>π</sub>2+<sub>σ</sub>2] Photocycloaddition Reactions of 2(1<i>H</i>)-Quinolones and Bicyclo[1.1.0]butanes作者:Morgane de Robichon、Thilo Kratz、Frederike Beyer、Julian Zuber、Christian Merten、Thorsten BachDOI:10.1021/jacs.3c08404日期:——enantioselectively to 2(1H)-quinolones upon irradiation (λ = 366 nm) in the presence of a chiral complexing agent. A two-point hydrogen bond between the quinolone and the template is responsible for stereocontrol in the photocycloaddition reaction. The reaction leads to the formation of products with a chiral bicyclo[2.1.1]hexane skeleton in high enantiomeric excess (91–99% ee). The chiral template can be almost在手性络合剂存在下,经照射 (λ = 366 nm),1-取代的双环[1.1.0]丁烷对映选择性地加成到 2(1 H )-喹诺酮类药物上。喹诺酮和模板之间的两点氢键负责光环加成反应中的立体控制。该反应导致形成具有高对映体过量(91-99% ee)的手性双环[2.1.1]己烷骨架的产物。手性模板几乎可以定量(97%)回收并用于另一个反应。可能存在三重态反应途径,如果反应要在可见光 (λ = 420 nm) 下进行,则敏化是一个合适的工具。
-
COMPOUNDS THAT ABROGATE THE CELL CYCLE G2 CHECKPOINT FOR USE IN THE TREATMENT OF CANCER申请人:CanBas Co., Ltd.公开号:EP3088397A1公开(公告)日:2016-11-02Substituted azole diones (II) and (III) are provided that kill cells, suppress cell proliferation, suppress cell growth, abrogate the cell cycle G2 checkpoint and/or cause adaptation to G2 cell cycle arrest. Methods of making the invention compounds are provided. The invention provides substituted azole diones (II) and (III) to treat cell proliferation disorders. The invention includes the substituted azole diones (II) and (III) for use to selectively kill or suppress cancer cells without additional anti-cancer treatment. The invention includes the cell cycle G2- checkpoint-abrogating substituted azole diones (II) and (III) for use to selectively sensitize cancer cells to DNA damaging reagents, treatments and/or other types of anti-cancer reagents.
-
Design, Synthesis, and Biological Evaluation of Quinoline (Quinolinone) Derivatives as NADPH Oxidase (NOX) Inhibitors作者:Lei Zhang、Xinliang Yang、Rui Yi、Siming Wu、Qianbin Li、Gaoyun Hu、Zhuo ChenDOI:10.1111/cbdd.14610日期:2024.8oxygen species. Recent studies have shown that NOXs is a very promising target for the treatment of diabetic nephropathy (DN). Here, a series of quinoline(quinolinone) derivatives have been designed based on pharmacophore strategy, synthesized and evaluated. Among them, 19d exhibits potent antiproliferative and NOXs inhibitory activities, and is worthy for further investigation.
表征谱图
-
氢谱1HNMR
-
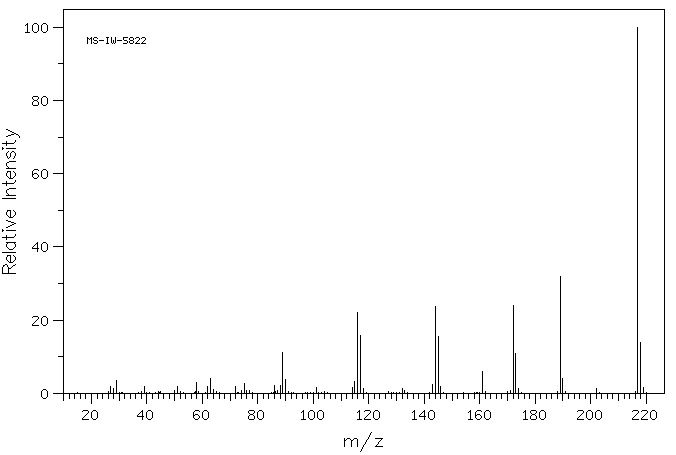
质谱MS
-
碳谱13CNMR
-
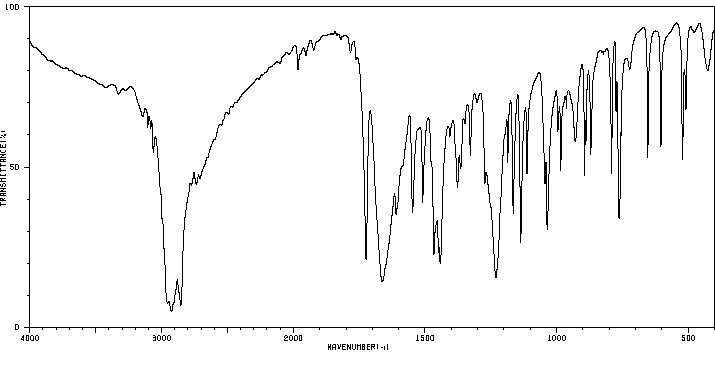
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43