叶醇 | 928-96-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:22.55°C (estimate)
-
沸点:156-157 °C(lit.)
-
密度:0.848 g/mL at 25 °C(lit.)
-
蒸气密度:3.45 (vs air)
-
闪点:112 °F
-
溶解度:二甲基亚砜:100 mg/mL(998.40 mM)
-
LogP:1 at 35℃
-
物理描述:Liquid
-
颜色/状态:Colorless liquid
-
气味:Powerful grassy-green odor
-
味道:Fresh, green, raw fruity with a pungent depth
-
蒸汽压力:0.94 mm Hg at 25 °C (est)
-
大气OH速率常数:1.10e-10 cm3/molecule*sec
-
稳定性/保质期:
- 常温常压下稳定,避免与强氧化剂和酸接触。
- 存在于烟叶中。
- 天然存在于柑橘类果汁、豆类、白兰地酒以及许多植物的叶子、精油和水果中。
-
折光率:Index of refraction: 1.4380 at 20 °C
-
解离常数:pKa: 16.8 at 25 °C (est)
-
保留指数:838;872;838;834;834;837;837;827;828;828;845;841;827;828;843;840.3;850;840;849;836;839;841;840;853;853;832;860;854;834;836;844;825;848;839;839;850;846;842;850;846;849;839;834;841;831;839.3;847;847;843;844;845;829;836;855;859;873;829;836;855;859;873;862;863;846;847;847;849;850;851;835;834;843;863;871;835;838;839;836;851;836;868;818;839;853;844;857;857;856;837;835;840;833;850;843;836;832;835;850;830;834;836;847;841.7;833;835;838;838;838;832;834;836;840;842;868;830;852;840;843;842;839;834;831;853;849;831;844;848;860;830;830;834;837;848;834;836;834;836;834;836;836;834;834;834;834;833;834;834;834;834;836;836;834;836;834;847;834;836;847;839;842;848;849;853;848;862;836;833
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F
-
安全说明:S16
-
危险类别码:R10
-
WGK Germany:1
-
海关编码:29052990
-
危险品运输编号:UN 1987 3/PG 3
-
危险类别:3
-
RTECS号:MP8400000
-
包装等级:III
-
储存条件:请将容器密封保存,并存放在阴凉、干燥的地方。
SDS
模块 1. 化学品
产品名称: cis-3-Hexen-1-ol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
急性毒性(经口) 第5级
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
吞咽可能有害。
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[急救措施] 若感不适:呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 顺-3-己烯-1-醇
百分比: >97.0%(GC)
CAS编码: 928-96-1
分子式: C6H12O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
顺-3-己烯-1-醇 修改号码:5
模块 4. 急救措施
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 157 °C
闪点: 62°C
爆炸特性
顺-3-己烯-1-醇 修改号码:5
模块 9. 理化特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.85
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: orl-rat LD50:4700 mg/kg
ipr-rat LD50:600 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: MP8400000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
顺-3-己烯-1-醇 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
叶醇是花香型及青香型香精的重要组成部分,仅需添加1%左右即可获得新鲜青叶香气。随着“回到大自然”的口号提出,叶醇将在西方型香精中发挥重要作用,并在瓜果香精中起到关键作用。这种无色液体具有优雅的青叶香气,微溶于水且易溶于乙醇、丙二醇等有机溶剂,在茶叶、番茄、桂花、茉莉、香叶、薄荷、紫罗兰等多种植物中常见。它的合成路线已有多种报道,如日本高砂香料公司以戊二烯和甲醛为原料制备叶醇。
应用叶醇广泛存在于绿色植物的叶、花及果实中,自古以来就被人体摄取。根据我国GB2760-1996标准规定,它可适量用于食品香精。在日本,叶醇被广泛应用于香蕉、草莓、柑桔、玫瑰香葡萄和苹果等天然风味香精的调配,并常与乙酸、戊酸及乳酸等酯类化合物共同使用,以改善食品口味,主要用以抑制清凉饮料和果汁的甜味余味。
在日用化工中的应用叶醇具有强烈的新鲜青草香气,是一种流行的清香型名贵香料。在香精生产中,叶醇及其酯类是不可或缺的调香剂。据统计,在世界上40多种著名香精配方中均含有叶醇成分。通常仅需添加0.5%或更少的比例,即可获得显著的青草香气。叶醇在化妆品行业中用于调配各种类似天然香料的人造精油,如铃兰型、丁香型、橡苔型、薄荷型和熏衣草型精油等,并可用来调制花香型香精,使人造精油和香精具有青香的头香香气。此外,叶醇还是合成茉莉酮和茉莉酮酸甲酯的重要原料,在20世纪60年代,它被誉为香料行业的绿色革命象征。
在生物防治中的应用叶醇不仅是植物和昆虫生理活性物质的关键成分,还被用作警报、集合或性激素。例如,将叶醇与苯琨按一定比例混合后可诱导雄性金龟子及甲虫聚集,从而实现对这些森林害虫的大规模捕杀。这进一步证明了叶醇在生物防治中的重要应用价值。
合成方法3-己炔-1-醇的合成通常采用两种方法:一种是在液氨中使金属锂或钠与乙炔反应生成乙炔(或乙炔钠),再与乙醇作用得到乙基乙炔;另一种则是以四氢呋喃为起始原料,通过选择性氢化或Wittig反应制得。
化学性质叶醇是一种无色油状液体,具有强烈的青草香气和新茶叶气息,沸点156℃,闪点44℃。它微溶于水但易溶于乙醇、丙二醇及大多数非挥发性油中。
用途GB2760—96将叶醇列为允许使用的食用香料之一,主要用于制备各种瓜果和薄荷型香精。顺式3-己烯醇具有类似异戊醇的香味,适用于高级香料的生产;反式3-已烯醇在稀释状态下有浓烈草味,适合特种香料的制作。
生产方法叶醇可通过多种化学反应合成。一种方法是在液氨中使用金属锂(或钠)与乙炔反应生成乙炔(或乙炔钠),再通过乙醇作用得到乙基乙炔;另一种则是以四氢呋喃为起始原料,进行选择性氢化或者Wittig反应制备。此外,还可以通过3-氯丙醇、三苯基膦和丙醛进行Wittig反应来合成叶醇。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 反式-3-己烯-1-醇 (E)-3-Hexen-1-ol 928-97-2 C6H12O 100.161 反式-2-已烯-1-醇 (2E)-hexen-1-ol 928-95-0 C6H12O 100.161 3-丁烯-1-醇 homoalylic alcohol 627-27-0 C4H8O 72.1069 正-3-己烯 cis-3-hexene 7642-09-3 C6H12 84.1613 顺式-3-己烯酸 (Z)-3-hexenoic acid 1775-43-5 C6H10O2 114.144 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 反式-3-己烯-1-醇 (E)-3-Hexen-1-ol 928-97-2 C6H12O 100.161 —— (Z)-hex-3-ene-1,6-diol 72530-33-7 C6H12O2 116.16 (3E)-3-己烯-1,6-二醇 (E)-3-hexen-1,6-diol 71655-17-9 C6H12O2 116.16 (Z)-1-甲氧基己-3-烯 (Z)-1-methoxyhex-3-ene 70220-06-3 C7H14O 114.188 (Z,z)-3,6-壬二烯-1-醇 (Z,Z)-3,6-nonadien-1-ol 53046-97-2 C9H16O 140.225 —— (3Z,6Z,9Z)-dodecatrien-1-ol 81345-02-0 C12H20O 180.29 —— (Z)-4-methylhex-3-en-1-ol 21019-60-3 C7H14O 114.188 —— (E)-4-methyl-3-hexen-1-ol 63714-11-4 C7H14O 114.188 顺-4-庚烯-1-醇 (Z)-4-hepten-1-ol 6191-71-5 C7H14O 114.188 —— 1-Vinyloxy-hex3(Z)-ene 366801-08-3 C8H14O 126.199 —— (3E,8Z)-tetradeca-3,8-dien-1-ol 177408-62-7 C14H26O 210.36 顺式-3-己烯酸 (Z)-3-hexenoic acid 1775-43-5 C6H10O2 114.144 反-2,顺6-壬二烯醇 (2E,6Z)-2,6-nonadien-1-ol 28069-72-9 C9H16O 140.225 —— (Z)-1-(allyloxy)hex-3-ene 70220-23-4 C9H16O 140.225 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Vacuum UV Photolyses of Some Bichromophoric Alkenes Possessing Hydroxyl or Methoxycarbonyl Group摘要:在185纳米下对一些天然存在的及相关的双色光烯进行直接光解,具有羟基或甲氧基碳酰基的烯烃在烯丙基、同烯丙基或远离位置的主要光产物为几何异构体,可通过气相色谱检测到。异构化产率对引入的官能团及其位置非常敏感;烯丙基烯醇的产率较低,而远离位置的功能化和羟基的酯化则提高了光异构化产率。DOI:10.1246/bcsj.58.2217
-
作为产物:参考文献:名称:[EN] SYNTHESIS OF OLEFINIC ALCOHOLS VIA ENZYMATIC TERMINAL HYDROXYLATION
[FR] SYNTHÈSE D'ALCOOLS OLÉFINIQUES PAR L'INTERMÉDIAIRE DE L'HYDROXYLATION TERMINALE ENZYMATIQUE摘要:在某些方面,本发明提供了一种通过将不饱和或饱和碳氢基质与羟化酶酶接触来生产末端羟基化烯烃和炔烃的方法。用于执行本发明方法的示例末端羟化酶对碳氢基质的一个末端碳具有很强的选择性,包括但不限于非血红素双铁脂烷单氧酶、细胞色素P450(例如,CYP52和CYP153家族的细胞色素P450)以及长链烷烃羟化酶。在某些实施例中,末端羟基化的烯烃或炔烃进一步转化为末端烯醛。在某些实施例中,末端羟基化的烯烃和炔烃可用作改变昆虫行为的昆虫信息素。在其他实施例中,末端羟基化的烯烃和炔烃是通过乙酰化或氧化醇基团来生产信息素的有用中间体。公开号:WO2015176020A1 -
作为试剂:参考文献:名称:Smoking compositions containing acylpyrazine ether flavorants摘要:在其一个实施例中,本发明提供了一种含有一种新型酰基吡嗪醚风味添加剂的吸烟组合物,例如1-(3-甲氧基-2-吡啶基)-2-甲基-1-丙酮。公开号:US04728738A1
文献信息
-
Functional alcohol releasing substance申请人:Kao Corporation公开号:US06486333B1公开(公告)日:2002-11-26This invention discloses a substance which is a betaine ester of a functional alcohol that has an amido bond in its molecule and releases the functional alcohol.这项发明揭示了一种物质,它是一种具有其分子中存在酰胺键的功能醇的甜菜碱酯,并释放出功能醇。
-
Iron-Catalyzed Direct Olefin Diazidation via Peroxyester Activation Promoted by Nitrogen-Based Ligands作者:Shou-Jie Shen、Cheng-Liang Zhu、Deng-Fu Lu、Hao XuDOI:10.1021/acscatal.8b00821日期:2018.5.4diazidation method previously developed by us. This method effectively addresses the limitations of the existing olefin diazidation methods. Most notably, previously problematic nonproductive oxidant decomposition can be minimized. Furthermore, X-ray crystallographic studies suggest that an iron–azide–ligand complex can be generated in situ from an iron acetate precatalyst and that it may facilitate peroxyester我们在此报告了一种铁催化的直接重氮化方法,该方法通过活化基于氮的配体促进的板凳稳定的过氧酯来实现。该方法对于广泛的烯烃和N-杂环是有效的,包括那些对于现有烯烃渗醛和重氮化方法而言是困难的底物的烯烃和N-杂环。值得注意的是,几乎化学计量的氧化剂和TMSN 3对于大多数基材来说,足以进行高产率的叠氮化。初步的机理研究阐明了该方法与我们先前开发的基于苯并二恶唑的烯烃重氮化方法之间的异同。该方法有效地解决了现有烯烃重氮化方法的局限性。最值得注意的是,以前有问题的非生产性氧化剂分解可以减至最少。此外,X射线晶体学研究表明,乙酸铁预催化剂可以原位生成叠氮化铁-配体配合物,并且在未重链烯烃的叠氮化过程中,它可以促进过氧酯的活化和决定速率的C–N 3键的形成。
-
[EN] SUBSTITUTED BUTANOL DERIVATIVES AND THEIR USE AS FRAGRANCE AND FLAVOR MATERIALS<br/>[FR] DÉRIVÉS SUBSTITUÉS DU BUTANOL, ET LEUR UTILISATION EN TANT QUE PARFUMS ET ARÔMES申请人:TAKASAGO PERFUMERY CO LTD公开号:WO2010002386A1公开(公告)日:2010-01-07The present invention is related to substituted butanol derivatives of the formula; wherein R is an unsubstituted or substituted C1-6 straight chain alkyl, an unsubstituted or substituted C3-6 branched chain alkyl an uosubstituted or substituted C3-6 straight chain alkenyl, an unsubstituted or substituted Cu= branched chain alkenyl, an unsubstituted or substituted C3-6 cycioalkyl, an unsubstituted or substituted C1-6 alkoxy, nitiile, halo, amino, an unsubstituted or substituted C1-6 alkyiamino, w. unsubstituted or substituted C1-6 dialkyfaraino, carboxy-C1-6 alkyiamino, carboxy-C1-6 di alkyl amino, an unsubstituted or substituted acetoxy, carboxy, an unsubstituted or substituted carboxyethyi, an unsubstituted or substituted C1-6 aikylcarbonyi, an unsubstituted or substituted C1-6 aikylcarboxy, ao unsubsiituted or substituted C1-6 alkylthio, an unsubstituted or substituted C1-6 alkyloxy. carboxamido, an unsubstituted or substituted C1-6 alkylcarboxarøido or an unsubstituted or substituted C1-6 dia'lkylcarboxamido. Such compounds are useful in flavor or flavor compositions.本发明涉及以下结构的取代丁醇衍生物;其中R是未取代或取代的C1-6直链烷基,未取代或取代的C3-6支链烷基,未取代或取代的C3-6直链烯基,未取代或取代的C3-6支链烯基,未取代或取代的C3-6环烷基,未取代或取代的C1-6烷氧基,硝基,卤素,氨基,未取代或取代的C1-6烷基氨基,未取代或取代的C1-6二烷基氨基,羧基-C1-6烷基氨基,羧基-C1-6二烷基氨基,未取代或取代的乙酰氧基,羧基,未取代或取代的羧乙基,未取代或取代的C1-6烷基羰基,未取代或取代的C1-6烷基羧基,未取代或取代的C1-6烷基硫基,未取代或取代的C1-6烷氧基,羧胺基,未取代或取代的C1-6烷基羧酰胺或未取代或取代的C1-6二烷基羧酰胺。这些化合物在香料或香料组合物中有用。
-
Olefin cyclisations of hindered α-acyliminium ions作者:B.P. Wijnberg、W.N. Speckamp、A.R.C. OostveenDOI:10.1016/0040-4020(82)85067-9日期:1982.1imides 2 and 4. HCOOH-Cyclisation of the hydroxylactams affords polycyclic piperidines through stereoselective α-acyliminium ring closure. Concomitant synchronous and stepwise cyclisation pathways are operative in the anti-periplanar addition of tertiary α-acyliminium ions to Me substituted olefins 8c and 11c.
-
THERAPEUTIC COMPOUNDS申请人:Hammond Gerald B.公开号:US20080188570A1公开(公告)日:2008-08-07The invention provides compounds of Formula (I): wherein R 1 and R 2 have any of the values or specific values defined herein, as well as compositions comprising such compounds and therapeutic methods comprising the administration of such compounds.本发明提供了式(I)的化合物: 其中R1和R2具有任意的或特定的定义值,以及包含这些化合物的组合物和包括这些化合物给药的治疗方法。
表征谱图
-
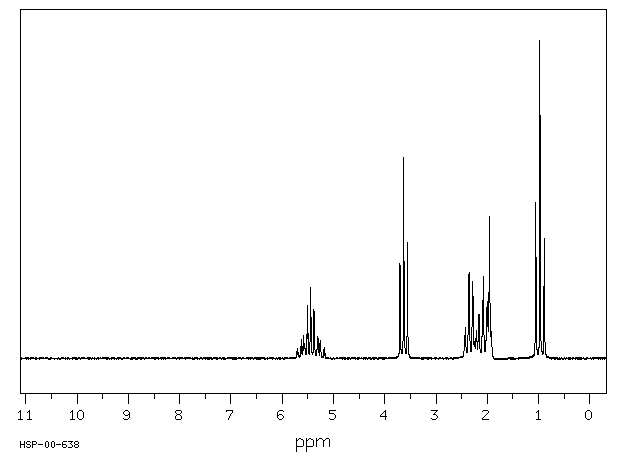
氢谱1HNMR
-
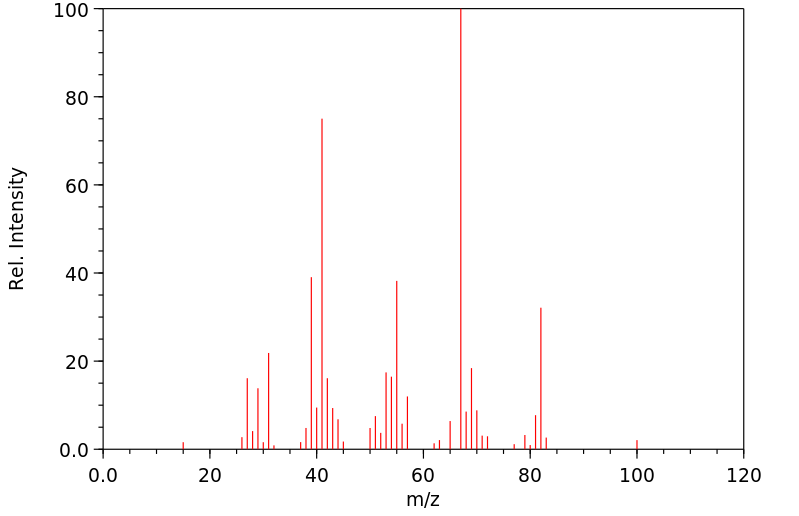
质谱MS
-
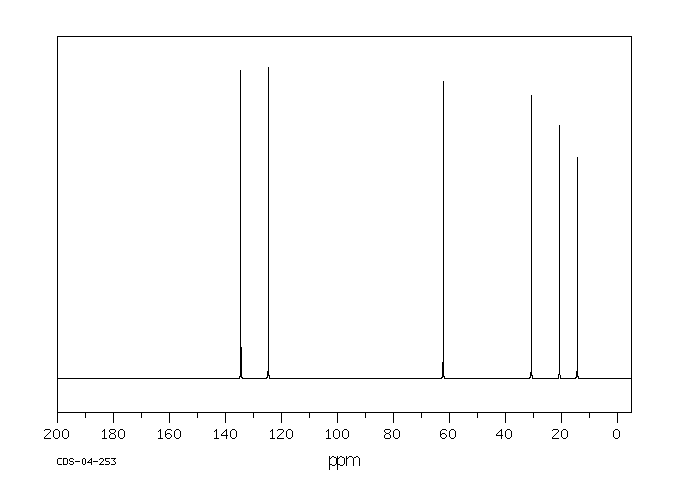
碳谱13CNMR
-
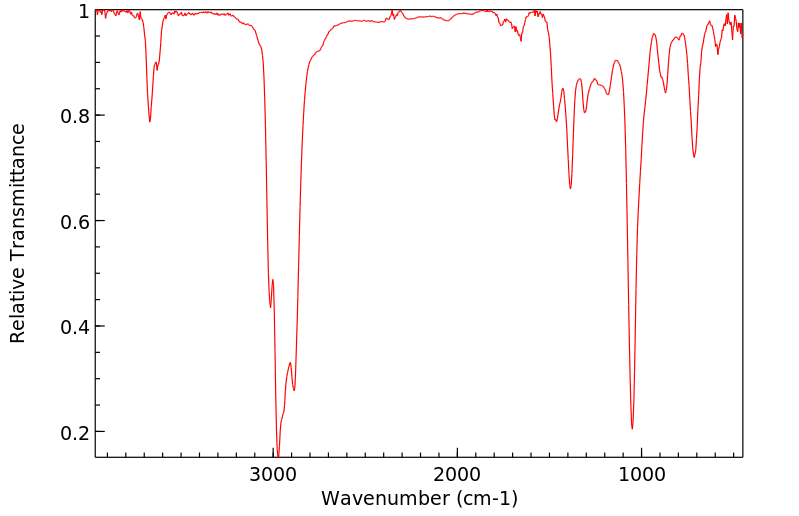
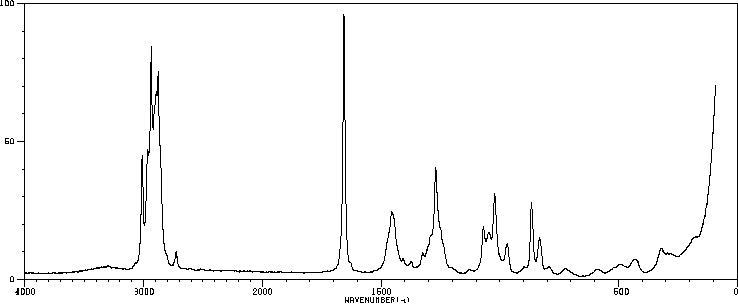
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息