5-溴-2-吡啶羧酸 | 30766-11-1
中文名称
5-溴-2-吡啶羧酸
中文别名
5-溴吡啶甲酸;2-羧酸-5-溴吡啶;5-溴-2-吡啶甲酸;2-羧基-5-溴吡啶;5-溴吡啶-2-羧酸;5-溴吡啶羧酸;5-溴-2-羧基吡啶;5-溴吡啶-2-甲酸
英文名称
5-bromoisonicotinic acid
英文别名
5-bromopicolinic acid;5-bromopyridine-2-carboxylic acid;5-bromopyridinecarboxylic acid;5-bromo-2-pyridinecarboxylic acid;5-bromo-pyridin-2-carboxylic acid;(5-bromopyridin-2-yl)carboxylic acid
CAS
30766-11-1
化学式
C6H4BrNO2
mdl
MFCD00234149
分子量
202.007
InChiKey
MNNQIBXLAHVDDL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:173-175°C
-
沸点:319.5±27.0 °C(Predicted)
-
密度:1.813±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇。
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,也未有已知危险反应。避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:50.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S24/25,S26,S36
-
危险类别码:R36/37/38
-
海关编码:2933399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气装置。
SDS
5-Bromopicolinic Acid Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 5-Bromopicolinic Acid
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 5-Bromopicolinic Acid
Percent: >97.0%(GC)(T)
CAS Number: 30766-11-1
Synonyms: 5-Bromo-2-pyridinecarboxylic Acid
Chemical Formula: C6H4BrNO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
5-Bromopicolinic Acid
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Slightly pale reddish yellow
Odor: No data available
pH: No data available
5-Bromopicolinic Acid
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:176 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
Soluble in : Methanol
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
5-Bromopicolinic Acid
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 5-Bromopicolinic Acid
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 5-Bromopicolinic Acid
Percent: >97.0%(GC)(T)
CAS Number: 30766-11-1
Synonyms: 5-Bromo-2-pyridinecarboxylic Acid
Chemical Formula: C6H4BrNO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
5-Bromopicolinic Acid
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Slightly pale reddish yellow
Odor: No data available
pH: No data available
5-Bromopicolinic Acid
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:176 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
Soluble in : Methanol
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
5-Bromopicolinic Acid
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
5-溴-2-吡啶羧酸用作医药化工中间体。
应用5-溴-2-吡啶羧酸是有机合成中间体和医药中间体,主要用于实验室有机合成过程和化工医药研发过程中。
制备方法以5-溴-2-甲基吡啶为原料,用水作为溶剂,在80°C的温度下加热。分批加入高锰酸钾,并维持反应温度在85-90°C之间,加热时间为60-100分钟。随后将反应物进行蒸馏、过滤并调节pH值,冷却后结晶得到5-溴-2-吡啶羧酸。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-溴吡啶-2-羧酸甲酯 methyl 5-bromopicolinate 29682-15-3 C7H6BrNO2 216.034 5-溴-2-嘧啶甲酸乙酯 ethyl 5-bromopicolinate 77199-09-8 C8H8BrNO2 230.061 5-溴吡啶甲酸叔丁酯 tert-butyl 5-bromopicolinate 845306-08-3 C10H12BrNO2 258.115 5-溴-2-羟甲基吡啶 (5-bromopyridin-2-yl)methanol 88139-91-7 C6H6BrNO 188.024 5-溴-2-吡啶甲醛 5-bromo-pyridine-2-carbaldehyde 31181-90-5 C6H4BrNO 186.008 5-氨基-2-吡啶羧酸 5-amino-pyridine-2-carboxylic acid 24242-20-4 C6H6N2O2 138.126 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-溴吡啶-2-羧酸甲酯 methyl 5-bromopicolinate 29682-15-3 C7H6BrNO2 216.034 5-溴-2-嘧啶甲酸乙酯 ethyl 5-bromopicolinate 77199-09-8 C8H8BrNO2 230.061 5-溴吡啶甲酸叔丁酯 tert-butyl 5-bromopicolinate 845306-08-3 C10H12BrNO2 258.115 5-溴-2-羟甲基吡啶 (5-bromopyridin-2-yl)methanol 88139-91-7 C6H6BrNO 188.024 3-溴-2-氯-6-吡啶羧酸 5-bromo-6-chloropicolinic acid 959958-25-9 C6H3BrClNO2 236.452 —— 5-bromopyridine-2-carboxylic acid benzyl ester 188052-14-4 C13H10BrNO2 292.132 —— 5-bromopicolinic (ethylcarbonic) anhydride 1415143-76-8 C9H8BrNO4 274.071 5-溴-6-氯吡啶-2-羧酸甲酯 methyl 5-bromo-6-chloropicolinate 1214353-79-3 C7H5BrClNO2 250.479 —— 3-bromo-6-methoxymethyl-pyridine-2-carboxylic acid 1316122-04-9 C8H8BrNO3 246.06 —— 3-Bromo-6-methoxymethyl-pyridine-2-carboxylic acid methyl ester 1316122-06-1 C9H10BrNO3 260.087 5-溴吡啶-2-甲酰氯 5-bromopyridine-2-carbonyl chloride 137178-88-2 C6H3BrClNO 220.453 1-(5-溴-2-吡啶)-乙醇 1-(5-bromopyridin-2-yl)ethanol 159533-68-3 C7H8BrNO 202.051 2-乙酰基-5-溴吡啶 1-(5-bromopyridin-2-yl)ethanone 214701-49-2 C7H6BrNO 200.035 5-溴吡啶甲酰胺 5-bromopicolinamide 90145-48-5 C6H5BrN2O 201.023 5-溴-2-羟甲基吡啶氮氧化物 (5-bromo-1-oxypyridin-2-yl)methanol 638219-84-8 C6H6BrNO2 204.023 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:[EN] N-ARYLMETHYL SULFONAMIDE NEGATIVE MODULATORS OF NR2A
[FR] MODULATEURS NÉGATIFS N-ARYLMÉTHYL SULFONAMIDE DE NR2A摘要:公开号:WO2015048503A3 -
作为产物:描述:参考文献:名称:Graf, Journal fur praktische Chemie (Leipzig 1954), 1932, vol. <2> 133, p. 36,49摘要:DOI:
-
作为试剂:描述:5-溴-2-氰基吡啶 、 sodium hydroxide 、 、 氨 、 溶剂黄146 在 丙酮 、 5-溴-2-吡啶羧酸 作用下, 以 乙醇 、 水 为溶剂, 反应 16.0h, 以to provide 5-bromopyridine-2-carboxylic acid (Compound B) as a solid的产率得到5-溴-2-吡啶羧酸参考文献:名称:Therapeutic agents useful for treating pain摘要:本发明公开了一个化合物的公式:其中Ar1,Ar2,X,Z1,Z2,R3和m如本文所述或其药学上可接受的盐(“吡啶烯化合物”);包括有效量的吡啶烯化合物的组合物;以及治疗或预防动物疼痛或其他病症的方法,包括向需要的动物投与有效量的吡啶烯化合物。公开号:US20060241117A1
文献信息
-
ANDROGEN RECEPTOR MODULATING CARBOXAMIDES申请人:Törmakängas Olli公开号:US20140094474A1公开(公告)日:2014-04-03Compounds of formula (I) or (II) wherein R x , R z , R 9 , R 10 , R 14 , R 14 ′, R 15 , R 15 ′, A and B are as defined in the claims and pharmaceutically acceptable salts and esters thereof, are disclosed. The compounds possess utility as tissue-selective androgen receptor modulators (SARM) and are useful as medicaments in the treatment of prostate cancer and other AR dependent conditions and diseases where AR antagonism is desired.式(I)或(II)的化合物 其中R x ,R z ,R 9 ,R 10 ,R 14 ,R 14 ′,R 15 ,R 15 ′,A和B如权利要求中所定义,并且其药学上可接受的盐和酯已被披露。这些化合物具有作为组织选择性雄激素受体调节剂(SARM)的效用,并且在治疗前列腺癌和其他依赖于AR的疾病和病症中,需要AR拮抗作用时可用作药物。
-
[EN] ANDROGEN RECEPTOR MODULATING CARBOXAMIDES<br/>[FR] CARBOXAMIDES MODULANT LES RÉCEPTEURS D'ANDROGÈNES申请人:ORION CORP公开号:WO2012143599A1公开(公告)日:2012-10-26Compounds of formula (I) wherein Rx, Rz, R9, R10, R14, R14', R15, R15', A and B are as defined in the claims and pharmaceutically acceptable salts and esters thereof, are disclosed. The compounds possess utility as tissue-selective androgen receptor modulators (SARM) and are particularly useful as medicaments in the treatment of prostate cancer and other AR dependent conditions and diseases where AR antagonism is desired.公式(I)中的化合物,其中Rx、Rz、R9、R10、R14、R14'、R15、R15'、A和B的定义如索赔中所述,并且其药学上可接受的盐和酯已被披露。这些化合物具有作为组织选择性雄激素受体调节剂(SARM)的效用,并且在治疗前列腺癌和其他依赖于雄激素受体的疾病和病症中特别有用,其中需要AR拮抗作用。
-
Substituted Heteroaromatic Pyrazole-Containing Carboxamide and Urea Compounds as Vanilloid Receptor Ligands申请人:FRANK Robert公开号:US20130029962A1公开(公告)日:2013-01-31Substituted heteroaromatic pyrazole-containing carboxamide and urea compounds as vanilloid receptor ligands, pharmaceutical compositions containing these compounds and also to a method of using these compounds for treating and/or inhibiting pain and further diseases and/or disorders.
-
[EN] N-CYCLOPROPYL-N-PIPERIDINYL-AMIDES, PHARMACEUTICAL COMPOSITIONS CONTAINING THEM AND USES THEREOF<br/>[FR] N-CYCLOPROPYL-N-PIPÉRIDINYL-AMIDES, COMPOSITIONS PHARMACEUTIQUES LES CONTENANT ET LEURS UTILISATIONS申请人:BOEHRINGER INGELHEIM INT公开号:WO2014019967A1公开(公告)日:2014-02-06The present invention relates to compounds of general formula (I), wherein R1, LP, HetAr1, (Het)Ar2 and n are as defined in the application, which have valuable pharmacological properties, and in particular bind to the GPR119 receptor and modulate its activity.本发明涉及一般式(I)的化合物,其中R1、LP、HetAr1、(Het)Ar2和n如申请中所定义,具有有价值的药理特性,特别是结合GPR119受体并调节其活性。
-
[EN] SUBSTITUTED BENZAMIDE DERIVATIVES<br/>[FR] DÉRIVÉS DE BENZAMIDE SUBSTITUÉS申请人:HOFFMANN LA ROCHE公开号:WO2011076678A1公开(公告)日:2011-06-30The invention relates to compounds of formula I wherein R is hydrogen or lower alkyl; R1 is -(CH2)n-(O)o-heterocycloalkyl or -C(O)-heterocycloalkyl, wherein the heterocycloalkyl group is optionally substituted by lower alkyl, hydroxy, halogen or by -(CH2)p-aryl; n is 0, 1 or 2; o is 0 or 1; p is 0, 1 or 2; R2 is CF3, cycloalkyl, optionally substituted by lower alkoxy or halogen, or is indan-2-yl, or is heterocycloalkyl, optionally substituted by heteroaryl, or is aryl or heteroaryl, wherein the aromatic rings are optionally substituted by one or two substituents, selected from lower alkyl, halogen, heteroaryl, hydroxy, CF3, OCF3, OCH2CF3, OCH2-cycloalkyl, OCH2C(CH2OH)(CH2C1)(CH3), S-lower alkyl, lower alkoxy, CH2-lower alkoxy, lower alkinyl or cyano, or by-C(O)-phenyl, -O-phenyl, -O- CH2-phenyl, phenyl or -CH2-phenyl, and wherein the phenyl rings may optionally be substituted by halogen, -C(O)-lower alkyl, -C(O)OH or -C(O)O-lower alkyl, or the aromatic rings are optionally substituted by heterocycloalkyl, OCH2-oxetan-3-yl or O-tetrahydropyran-4-yl, optionally substituted by lower alkyl; X is a bond, -NR'-, -CH2NH-, -CHR''-, -(CHR'')q-O-, -O-(CHR'')q- or -(CH2)2-; Y is a bond or -CH2- R' is hydrogen or lower alkyl, R'' is hydrogen, lower alkyl, CF3, lower alkoxy, q is 0, 1, 2 or 3; or to a pharmaceutically suitable acid addition salt thereof. It has now been found that the compounds of formula I have a good affinity to the trace amine associated receptors (TAARs), especially for TAAR1. The compounds may be used for the treatment of depression, anxiety disorders, bipolar disorder, attention deficit hyperactivity disorder (ADHD), stress-related disorders, psychotic disorders such as schizophrenia, neurological diseases such as Parkinsons disease, neurodegenerative disorders such as Alzheimers disease, epilepsy, migraine, hypertension, substance abuse and metabolic disorders such as eating disorders, diabetes, diabetic complications, obesity, dyslipidemia, disorders of energy consumption and assimilation, disorders and malfunction of body temperature homeostasis, disorders of sleep and circadian rhythm, and cardiovascular disorders.该发明涉及以下式I的化合物,其中R是氢或较低的烷基;R1是-(CH2)n-(O)o-杂环烷基或-C(O)-杂环烷基,其中杂环烷基基团可选择地被较低的烷基,羟基,卤素或-( )p-芳基取代;n为0、1或2;o为0或1;p为0、1或2;R2为CF3,环烷基,可选择地被较低的烷氧基或卤素取代,或为茚-2-基,或为杂环烷基,可选择地被杂芳基取代,或为芳基或杂芳基,其中芳香环可选择地被来自较低的烷基,卤素,杂芳基,羟基, ,O ,O ,O -环烷基,O C( OH)( C1)(CH3),S-较低的烷基,较低的烷氧基, -较低的烷氧基,较低的炔基或氰基,或-C(O)-苯基,-O-苯基,-O- -苯基,苯基或- -苯基选择的一个或两个取代基取代,其中苯环可选择地被卤素,-C(O)-较低的烷基,-C(O)OH或-C(O)O-较低的烷基取代,或芳香环可选择地被杂环烷基,O -氧杂环戊烷-3-基或O-四氢吡喃-4-基,可选择地被较低的烷基取代;X为键,-NR'-,- NH-,-CHR''-,-(CHR'')q-O-,-O-(CHR'')q-或-( )2-;Y为键或- -;R'为氢或较低的烷基,R''为氢,较低的烷基, ,较低的烷氧基,q为0、1、2或3;或其药学上适宜的酸盐。现已发现,该式I的化合物对痕量胺相关受体(TAARs)具有良好的亲和力,特别是对于TAAR1。这些化合物可用于治疗抑郁症,焦虑症,躁郁症,注意力缺陷多动障碍(ADHD),与压力有关的疾病,如精神分裂症,帕金森病等神经疾病,阿尔茨海默病等神经退行性疾病,癫痫,偏头痛,高血压,物质滥用和代谢性疾病,如进食障碍,糖尿病,糖尿病并发症,肥胖症,血脂异常,能量消耗和吸收异常,体温稳态异常,睡眠和昼夜节律异常,以及心血管疾病。
表征谱图
-
氢谱1HNMR
-
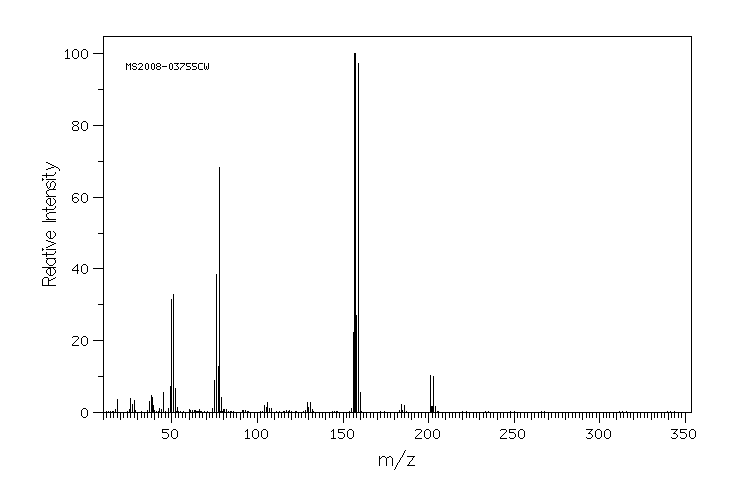
质谱MS
-
碳谱13CNMR
-
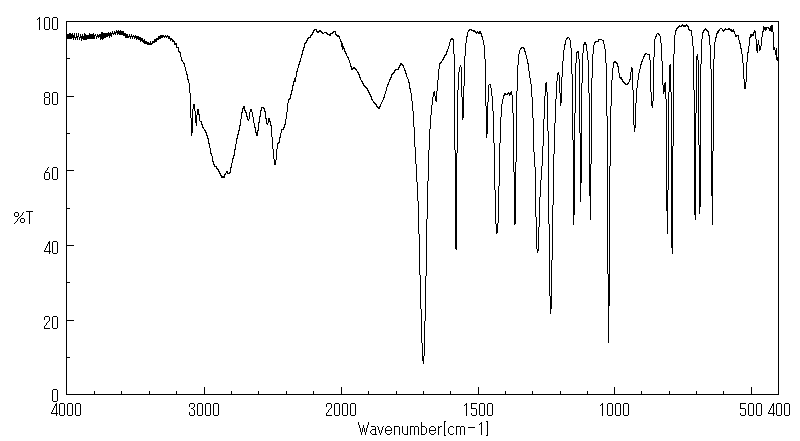
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-