sodium cholate | 361-09-1
分子结构分类
中文名称
——
中文别名
——
英文名称
sodium cholate
英文别名
cholic acid sodium salt;sodium;(4R)-4-[(3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-3,7,12-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoate
CAS
361-09-1
化学式
C24H39O5*Na
mdl
——
分子量
430.56
InChiKey
NRHMKIHPTBHXPF-TUJRSCDTSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>225°C (dec.)
-
比旋光度:35 º (c=0.6, ethanol)
-
密度:0.999 at 20.09℃
-
溶解度:可溶于DMSO(轻微)、乙醇(轻微、超声处理)、甲醇(轻微)、水
-
LogP:4.3 at 22℃ and pH5.7
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-0.88
-
重原子数:30
-
可旋转键数:4
-
环数:4.0
-
sp3杂化的碳原子比例:0.96
-
拓扑面积:101
-
氢给体数:3
-
氢受体数:5
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29173990
-
RTECS号:FZ9600000
-
储存条件:棕色玻璃瓶密封包装,在低温下存储。
SDS
| Name: | Cholic acid sodium salt 99% (Titr.) Material Safety Data Sheet |
| Synonym: | Sodium cholate; 3alpha,7alpha,12alpha-Trihydroxy-5-cholanic acid sodium salt; Cholic acid, monosodium salt, sodium cholic acid; Cholan-24-oic acid, 3,7,12-trihydroxy-, monosodium salt, (3-alpha,5-beta,7-alpha; Cholic acid, monosodium salt; D |
| CAS: | 361-09-1 |
Synonym:Sodium cholate; 3alpha,7alpha,12alpha-Trihydroxy-5-cholanic acid sodium salt; Cholic acid, monosodium salt, sodium cholic acid; Cholan-24-oic acid, 3,7,12-trihydroxy-, monosodium salt, (3-alpha,5-beta,7-alpha; Cholic acid, monosodium salt; D
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 361-09-1 | Cholic acid sodium salt | 99 | 206-643-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Hygroscopic (absorbs moisture from the air).The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation. May cause effects similar to those described for ingestion.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Get medical aid immediately. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 361-09-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 198 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Soluble.
Specific Gravity/Density: Not available.
Molecular Formula: C24H39NaO5
Molecular Weight: 430.55
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants, exposure to moist air or water.
Incompatibilities with Other Materials:
Oxidizing agents, moisture.
Hazardous Decomposition Products:
Acrid smoke and fumes.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 361-09-1: FZ9600000 LD50/LC50:
CAS# 361-09-1: Oral, mouse: LD50 = 2400 mg/kg.
Carcinogenicity:
Cholic acid sodium salt - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 361-09-1: 1
Canada
CAS# 361-09-1 is listed on Canada's DSL List.
CAS# 361-09-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 361-09-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:sodium cholate 在 Pseudomonas fluorescens B26 broth 作用下, 以 水 为溶剂, 反应 48.0h, 以41%的产率得到去氢胆酸参考文献:名称:Regioselective microbial oxidation of bile acids摘要:High regioselectivity in the microbial oxidation of C-7, C-3 and C-12 hydroxyl groups of cholic, chenodeoxycholic, deoxycholic and hyocholic acids 1-4 is reported. The tested microrganisms have been isolated from 50 enviromental samples withdrawed from an industry that extracts and purify bile acids. (C) 1998 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4020(97)10408-2
-
作为产物:描述:参考文献:名称:胆汁酸的羟基-双膦酸类似物的合成,表征和生物学活性摘要:现在,双膦酸盐(BPs)是用于与骨吸收增加有关的疾病(例如骨质疏松症和肿瘤性骨病)的最广泛使用的药物。BP的一个显着缺点是口服吸收差,双膦酸盐骨架中胆汁酸取代基的存在会增强它们的口服吸收,而没有毒性作用。提出了含胆汁酸的羟基双膦酸酯的直接合成方法和这些药学上重要分子的完整表征,包括亲和力评估和与羟基磷灰石的结合机理。使用中性红测定法在L929细胞系和破骨细胞的原代培养物中测定了含胆汁酸的双膦酸盐的生物活性。发现新化合物的生物活性优于已确定活性的双膦酸酯。DOI:10.1016/j.ejmech.2012.03.020
-
作为试剂:参考文献:名称:O'Connor, Charmian J.; Wallace, Robert G., Australian Journal of Chemistry, 1984, vol. 37, # 9, p. 1881 - 1893摘要:DOI:
文献信息
-
Pharmaceutical compositions of drug-oligomer conjugates and methods of treating diseases therewith申请人:——公开号:US20030069170A1公开(公告)日:2003-04-10Pharmaceutical compositions that include a drug-oligomer conjugate, a fatty acid component, and a bile salt component are described. The drug is covalently coupled to an oligomeric moiety. The fatty acid component and the bile salt component are present in a weight-to-weight ratio of between 1:5 and 5:1. Methods of treating diseases in a subject in need of such treatment using such pharmaceutical compositions are also provided, as are methods of providing such pharmaceutical compositions.
-
BORON-CONTAINING SMALL MOLECULES申请人:Xia Yi公开号:US20100256092A1公开(公告)日:2010-10-07This invention relates to, among other items, 6-substituted benzoxaborole compounds and their use for treating bacterial infections.这项发明涉及6-取代苯硼酯化合物等物品,以及它们用于治疗细菌感染的用途。
-
[EN] ANALGESIC THAT BINDS FILAMIN A<br/>[FR] ANALGÉSIQUE QUI SE LIE À LA FILAMINE A申请人:PAIN THERAPEUTICS INC公开号:WO2010051374A1公开(公告)日:2010-05-06A compound, composition and method are disclosed that can provide analgesia. A contemplated compound has a structure that corresponds to Formula A, wherein A, B, X, R1, R2, R7 and R8, and the dashed lines are defined within.公开了一种化合物、组合物及方法,能够提供镇痛效果。所考虑的化合物具有与式A相对应的结构,其中A、B、X、R1、R2、R7和R8以及虚线均在定义范围内。
-
[EN] PROSTATE-SPECIFIC MEMBRANE ANTIGEN ANTIBODY DRUG CONJUGATES<br/>[FR] CONJUGUÉS ANTICORPS-MÉDICAMENT D'ANTIGÈNE MEMBRANAIRE SPÉCIFIQUE À LA PROSTATE申请人:AMBRX INC公开号:WO2013185117A1公开(公告)日:2013-12-12This invention relates to prostate-specific membrane antigen (PSMA) antibodies and antibody drug conjugates comprising at least one non-naturally-encoded amino acid. Disclosed herein are αPSMA antibodies with one or more non-naturally encoded amino acids and further disclosed are antibody drug conjugates wherein the αPSMA antibodies of the invention are conjugated to one or more toxins. Also disclosed herein are non-natural amino acid dolastatin analogs that are further modified post-translationally, methods for effecting such modifications, and methods for purifying such dolastatin analogs. Typically, the modified dolastatin analogs include at least one oxime, carbonyl, dicarbonyl, and/or hydroxylamine group. Further disclosed are methods for using such non-natural amino acid antibody drug conjugates, dolastatin analogs, and modified non-natural amino acid dolastatin analogs, including therapeutic, diagnostic, and other biotechnology uses.
-
[EN] 3-ARYLOXY AND 3-HETEROARYLOXY SUBSTITUTED BENZO(B) THIOPHENES AS THERAPEUTIC AGENTS WITH PI3K ACTIVITY<br/>[FR] BENZO[B]THIOPHENES A SUBSTITUTION 3-ARYLOXY ET 3-HETEROARYLOXY EN TANT QU'AGENTS THERAPEUTIQUES A ACTIVITE PI3K申请人:WARNER LAMBERT CO公开号:WO2004108715A1公开(公告)日:2004-12-16The present invention provides benzo[b]thiophenes of Formula (I), wherein R1, R2, R3, R4, R5, and L have any of the values defined therefor in the specification, and pharmaceutically acceptable salts thereof, that are useful as agents in the treatment of diseases and conditions, including inflammatory diseases, cardiovascular diseases, and cancers. Also provided are pharmaceutical compositions comprising one or more compounds of Formula (I).本发明提供了式(I)的苯并[b]噻吩,其中R1、R2、R3、R4、R5和L具有规范中为其定义的任何值,以及药用可接受的盐,这些盐作为治疗疾病和状况的药剂是有用的,包括炎性疾病、心血管疾病和癌症。另外还提供了包含一个或多个式(I)化合物的药物组合物。
表征谱图
-
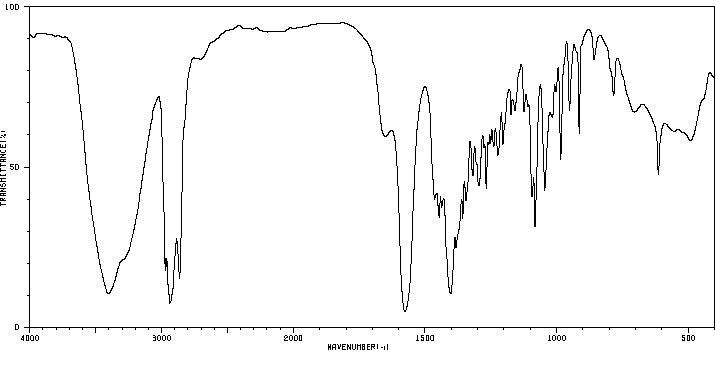
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
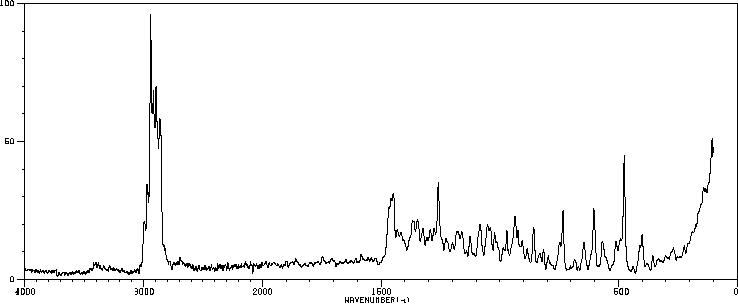
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B