3-氯苯硫酚 | 2037-31-2
中文名称
3-氯苯硫酚
中文别名
间氯苯硫酚;3-氯硫酚
英文名称
3-chlorophenylthiol
英文别名
3-Chlorothiophenol;3-chlorobenzenethiol
CAS
2037-31-2
化学式
C6H5ClS
mdl
MFCD00004839
分子量
144.625
InChiKey
CQJDYPZUDYXHLM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:298.7-304.3 °C (decomp)
-
沸点:110 °C30 mm Hg(lit.)
-
密度:1.245 g/mL at 25 °C(lit.)
-
闪点:194 °F
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也未有已知危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:T
-
危险等级:8
-
危险品标志:C
-
安全说明:S23,S26,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2930909090
-
危险品运输编号:UN 1760 8/PG 2
-
包装等级:III
-
危险类别:8
-
危险标志:GHS05
-
危险性描述:H314
-
危险性防范说明:P280,P305 + P351 + P338,P310
-
储存条件:请将贮藏器密封存放于阴凉干燥处,并确保工作环境有良好的通风或排气设施。
SDS
| Name: | 3-Chlorothiophenol 97% Material Safety Data Sheet |
| Synonym: | 3-Chlorobenzenethiol; Benzenthiol, 3-Chloro-; M-Chlorothiophenol |
| CAS: | 2037-31-2 |
Synonym:3-Chlorobenzenethiol; Benzenthiol, 3-Chloro-; M-Chlorothiophenol
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2037-31-2 | 3-Chlorothiophenol | 97 | 218-010-0 |
Risk Phrases: 23/24/25 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.Stench.Lachrymator (substance which increases the flow of tears).
Potential Health Effects
Eye:
Causes eye irritation. Lachrymator (substance which increases the flow of tears). May cause chemical conjunctivitis.
Skin:
Causes skin irritation. May be fatal if absorbed through the skin.
Substance is readily absorbed through the skin.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 2037-31-2: Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible. Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure. Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin. Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Wear a NIOSH/MSHA or European Standard EN 149 approved full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear almost colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 110 deg C @ 30.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 90 deg C ( 194.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.2450g/cm3
Molecular Formula: C6H5ClS
Molecular Weight: 144.62
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures. Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, oxides of sulfur, carbon dioxide, hydrogen sulfide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2037-31-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Chlorothiophenol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
WGK (Water Danger/Protection)
CAS# 2037-31-2: No information available.
Canada
CAS# 2037-31-2 is listed on Canada's NDSL List.
CAS# 2037-31-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2037-31-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质:无色透明至淡黄色的液体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (3-氯苯基)硫氰酸盐 1-chloro-3-thiocyanatobenzene 2402-00-8 C7H4ClNS 169.634 3,3’-二氯二苯二硫醚 bis(3-chlorophenyl) disulfide 19742-92-8 C12H8Cl2S2 287.234 —— Ethyl-3-chlorphenyl-Disulfid 55975-72-9 C8H9ClS2 204.744 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-氯茴香硫醚 1-chloro-3-methylsulfanylbenzene 4867-37-2 C7H7ClS 158.652 间氯苯磺酰氯 m-chlorobenzenesulfenyl chloride 13733-68-1 C6H4Cl2S 179.07 —— (3-chlorophenyl)(ethyl)sulfane 34126-15-3 C8H9ClS 172.678 (3-氯苯基)硫氰酸盐 1-chloro-3-thiocyanatobenzene 2402-00-8 C7H4ClNS 169.634 —— 3-chlorophenyl vinyl sulfide 42150-16-3 C8H7ClS 170.663 1-氯-3-苯基硫基苯 m-chlorophenyl phenylsulfide 38700-88-8 C12H9ClS 220.722 3,3’-二氯二苯二硫醚 bis(3-chlorophenyl) disulfide 19742-92-8 C12H8Cl2S2 287.234 —— 3-chlorophenyl isopropyl sulfide 55698-06-1 C9H11ClS 186.705 —— (3-chlorophenyl)(propyl)sulfide 1713160-58-7 C9H11ClS 186.705 —— allyl(3-chlorophenyl)sulfane 477983-30-5 C9H9ClS 184.689 —— 2-((3-chlorophenyl)thio)acetonitrile —— C8H6ClNS 183.661 —— 1-(3-chlorophenylthio)-2-propyne —— C9H7ClS 182.674 —— (2-bromoethyl)(3-chlorophenyl)sulfane 3983-71-9 C8H8BrClS 251.575 —— 1-chloro-2-(3-chlorobenzenethio)ethane —— C8H8Cl2S 207.124 —— (3-chlorophenyl)(difluoromethyl)sulfane —— C7H5ClF2S 194.633 —— Ethyl-3-chlorphenyl-Disulfid 55975-72-9 C8H9ClS2 204.744 —— 3,4'-dichlorodiphenylsulfide 6875-99-6 C12H8Cl2S 255.168 - 1
- 2
反应信息
-
作为反应物:描述:3-氯苯硫酚 在 ammonium hydroxide 、 potassium tert-butylate 作用下, 以 乙醇 、 二甲基亚砜 为溶剂, 反应 1.0h, 生成 2-Amino-6-(3-chloro-phenylsulfanyl)-benzonitrile参考文献:名称:2-氨基-6-芳基磺酰基苯甲腈作为HIV-1的非核苷类逆转录酶抑制剂。摘要:发现一系列2-氨基-5-芳基硫代苄腈(1)具有抗HIV-1的活性。结构上的改变产生了亚砜(2)和砜(3)。亚砜通常显示出与HIV-1相似的抗病毒活性。然而,砜是最有效的类似物系列,其中一些具有在纳摩尔范围内的抗HIV-1活性。结构活性关系(SAR)研究表明,间位取代基,特别是间位甲基取代基,总是会增加抗病毒活性。然而,最佳的抗病毒活性由芳基磺酰基部分中的两个间位基团都被取代且取代基之一是甲基的化合物表现出来。这种混乱导致化合物3v,3w,3x和3y在低纳摩尔范围内具有针对HIV-1的IC50值。当评估它们对关键的非核苷类逆转录酶抑制剂(NNRTI)相关突变体的广谱抗病毒活性时,所有二元取代的砜3u-z和2-萘基类似物3ee通常显示出对数显性的单摩尔纳米摩尔活性。 V106A和P236L菌株以及针对菌株E138K,V108I和Y188C的亚微摩尔至低纳摩尔活性。然而,他们显示出缺乏针对K10DOI:10.1021/jm0004906
-
作为产物:描述:参考文献:名称:Nucleophilic cleavage of the sulfur-sulfur bond by phosphorus nucleophiles. Kinetic study of the reduction of aryl disulfides with triphenylphosphine and water摘要:DOI:10.1021/ja00826a021
-
作为试剂:描述:sodium 3-chloro-thiophenolate 在 3-氯苯硫酚 作用下, 以 甲醇 为溶剂, 反应 1.17h, 生成 2-(3-chlorophenylthio)-4-nitrothiophene参考文献:名称:取代基对甲苯中反式-2,3-双(芳硫基)-4-硝基-2,3-二氢噻吩β-消除槟榔酚作用机理的动力学研究摘要:2,3-二氢噻吩衍生物(1b - f)在甲苯中容易进行区域特异性的三丁胺促进的槟榔酚合成消除,从而得到2-(芳硫基)-4-硝基噻吩(2b - f)。对于系列中每个成员显示的相当复杂的动力学行为,基于底物的共轭碱的稳定性提出了合理化建议,该稳定性足以允许沿着反应坐标形成不可忽略的中间离子对浓度。该系统可以得出有关离去基团驱除步骤的直接结论:可以假定在过渡态下碳与离去基团之间键断裂的高级程度。DOI:10.1039/p29850001741
文献信息
-
[EN] BENZAMIDE OR BENZAMINE COMPOUNDS USEFUL AS ANTICANCER AGENTS FOR THE TREATMENT OF HUMAN CANCERS<br/>[FR] COMPOSÉS BENZAMIDE OU BENZAMINE À UTILISER EN TANT QU'ANTICANCÉREUX POUR LE TRAITEMENT DE CANCERS HUMAINS申请人:UNIV TEXAS公开号:WO2017007634A1公开(公告)日:2017-01-12The described invention provides small molecule anti-cancer compounds for treating tumors that respond to cholesterol biosynthesis inhibition. The compounds selectively inhibit the cholesterol biosynthetic pathway in tumor-derived cancer cells, but do not affect normally dividing cells.
-
THIOXANTHONE RING SYSTEM DERIVATIVES申请人:HUANG Hsu-Shan公开号:US20120088810A1公开(公告)日:2012-04-12A thioxanthone ring system derivative compound is provided. The thioxanthone ring system derivative compound is represented by a formula (I): wherein X is a substituent being one selected from a group consisting of halogens, wherein R 1 is a substituent being one selected from a group consisting of sulfur and sulfur dioxide, wherein R 2 is a substituent being one selected from a group consisting of C 1 ˜C 10 alkyl group, C 3 ˜C 10 branched alkyl group, C 3 ˜C 10 cyclic alkyl group, phenyl group, phenyl alkyl group, and wherein hydrogen of phenyl group can be partially substituted by halogens, alkoxyl group, C 1 ˜C 10 alkyl group, nitro group or amine group.
-
一种环庚三烯-1-硫醚类化合物及其合成方法和应用申请人:广东工业大学公开号:CN109942465A公开(公告)日:2019-06-28
-
6-Arylthio-3-hydroxypyrimidine-2,4-diones potently inhibited HIV reverse transcriptase-associated RNase H with antiviral activity作者:Lei Wang、Jing Tang、Andrew D. Huber、Mary C. Casey、Karen A. Kirby、Daniel J. Wilson、Jayakanth Kankanala、Jiashu Xie、Michael A. Parniak、Stefan G. Sarafianos、Zhengqiang WangDOI:10.1016/j.ejmech.2018.07.039日期:2018.8Human immunodeficiency virus (HIV) reverse transcriptase (RT) associated ribonuclease H (RNase H) remains the only virally encoded enzymatic function not targeted by current drugs. Although a few chemotypes have been reported to inhibit HIV RNase H in biochemical assays, their general lack of significant antiviral activity in cell culture necessitates continued efforts in identifying highly potent人类免疫缺陷病毒(HIV)逆转录酶(RT)相关的核糖核酸酶H(RNase H)仍然是当前药物未靶向的唯一病毒编码的酶功能。尽管在生化分析中已经报道了一些化学型抑制HIV RNase H,但是它们在细胞培养中普遍缺乏显着的抗病毒活性,因此需要继续努力鉴定具有高效抗病毒活性的RNase H抑制剂。我们在此报告了3-羟基嘧啶-2,4-二酮(HPD)化学型的新6-芳硫基亚型的设计,合成,生化和抗病毒评估。在生化分析中,这些新的类似物在单个纳摩尔范围内抑制RT RNase H,而在浓度高达10μM的情况下却不抑制RT聚合酶(pol),具有非凡的生化抑制选择性。许多类似物还在低至亚微摩尔范围内抑制整合酶链转移(INST)活性。更重要的是,大多数类似物都在低微摩尔范围内抑制HIV而无细胞毒性。最后,复合13j(RNase H IC 50 = 0.005μM; RT pol IC 50 = 10μM;
-
Cell adhesion-inhibiting antiinflammatory and immune-suppressive compounds申请人:Abbott Laboratories公开号:US20040116518A1公开(公告)日:2004-06-17The present invention relates to novel cinnamide compounds that are useful for treating inflammatory and immune diseases and cerebral vasospasm, to pharmaceutical compositions containing these compounds, and to methods of inhibiting inflammation or suppressing immune response in a mammal.本发明涉及新型肉桂酰胺化合物,用于治疗炎症和免疫性疾病以及脑血管痉挛,以及含有这些化合物的药物组合物,以及在哺乳动物中抑制炎症或抑制免疫反应的方法。
表征谱图
-
氢谱1HNMR
-
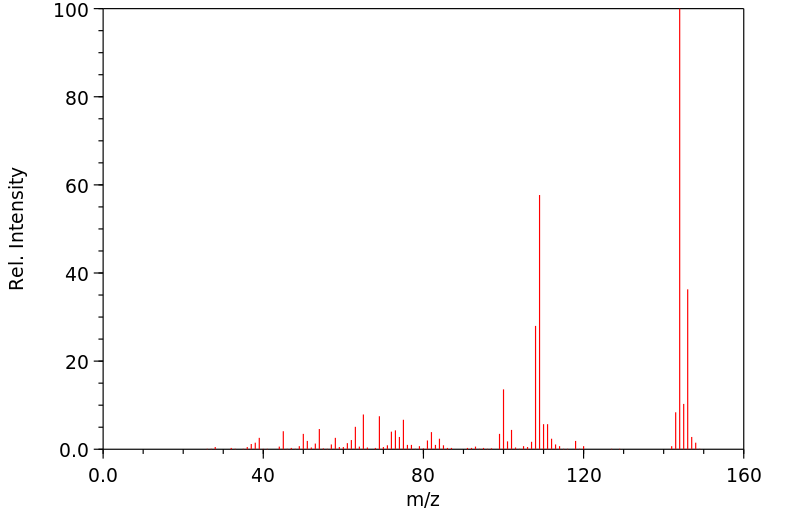
质谱MS
-
碳谱13CNMR
-
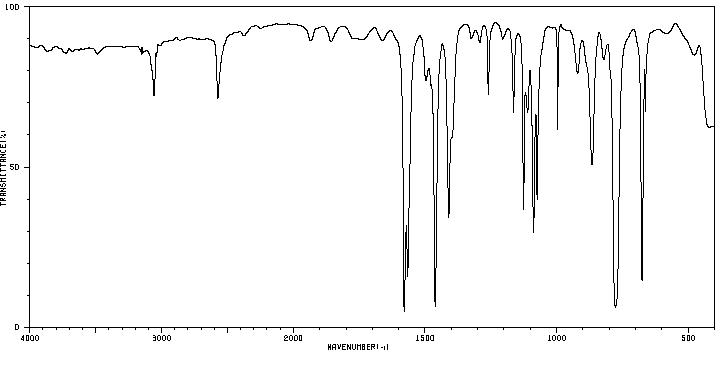
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
邻氯苯硫酚
邻巯基苯乙酮肟
苯硫醇,4-氨基-2,5-二氟-
苯硫醇,2-[(丙基硫代)甲基]-
苯硫醇,2-(氨基甲基)-6-氟-
苯硫醇
苯硫酚钾
苯硫酚钠
苯硫酚
苯六硫酚
甲苯-3,4-二硫酚
烯丙基(邻巯基苯基)甲基硫醚
戊甲基苯硫醇
对氟苯硫酚
对叔丁基硫酚
对-(三甲基甲硅烷)苯硫酚
四巯基苯
五氯苯硫酚锌盐
五氯苯硫酚
五氟苯硫酚
三(巯基苯基)(甲基)硅烷
S-(2-溴-2-氯-1,1-二氟乙基)半胱氨酸
6-氨基-2-氟-3-甲基苯硫醇
6-氨基-2,3-二氟苯硫醇
5-溴-1,3-苯基二硫醇
5-氯-2-甲基苯硫酚
5-氯-2-(甲硫基)苯硫酚
5-氨基-2-氯-4-氟苯硫醇
5-氟-2-甲氧基苯硫醇
5-氟-2-甲基硫代苯酚
5-氟-2-巯基苄醇
4H-吡喃-4-酮,2,3-二氢-2-甲基-,(2R)-(9CI)
4-辛氧基苯硫醇
4-羟基苯硫醇钠
4-羟基苯硫酚
4-羟-3-甲基苯硫酚
4-碘代苯-1-硫醇
4-甲苯硫酚
4-甲硫基苯硫醇
4-甲氧基苯硫酚
4-甲氧基-3-<(2-甲氧基吡啶-5-基)甲基>苯硫酚
4-甲氧基-2-硫基苯甲醛
4-甲氧基-2-甲基硫代苯酚
4-甲基苯硫醇铅
4-甲基磺酰氧基苯硫酚
4-甲基-2-硫基苯甲醛
4-甲基-2,3,5,6-四氟苯硫酚
4-环戊基苯硫醇
4-环己基-苯硫酚
4-环丙基苯硫醇