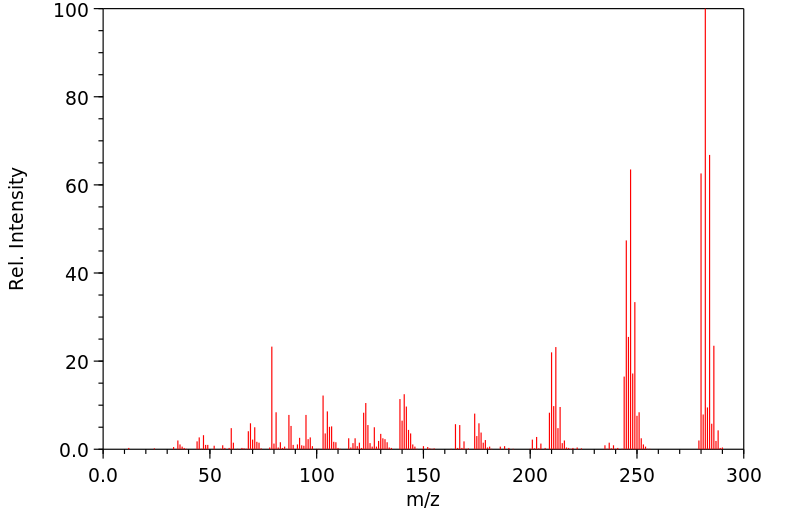
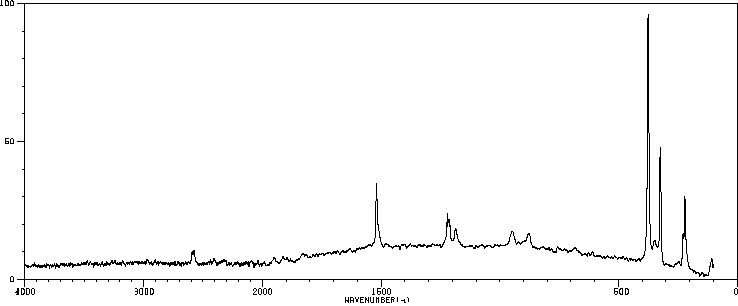
代谢
雌性大鼠通过腹腔注射了14C-六氯苯。药物在2到3次给药。总剂量分别为260和390 mg/kg的14C-六氯苯。在第一次注射后的4周内收集了动物的尿液和粪便。检查了排泄物和动物的一些组织的放射性含量以及六氯苯及其代谢物。... 在尿液中,五氯酚、四氯对氢醌和五氯硫酚作为主要代谢物存在。四氯硫酚的一个异构体作为次要代谢物存在。在粪便中仅鉴定出了五氯酚和五氯硫酚。
Female rats were dosed intraperitoneally with 14C-hexachlorobenzene. The drug was administered on 2 or 3 occasions. The total doses amounted to 260 and 390 mg/kg 14C-hexachlorobenzene, respectively. Urine and feces from the animals were collected over a period of 4 wk after the first injection. Both excreta and some tissues of the animals were examined for their content of radioactivity and for hexachlorobenzene and its metabolites. ... In urine pentachlorophenol, tetrachlorohydroquinone, and pentachlorothiophenol were present as major metabolites. One of the isomers of tetrachlorothiophenol was present as a minor metabolite. In the feces pentachlorophenol and pentachlorothiophenol only were identified ...
来源:Hazardous Substances Data Bank (HSDB)