DL-Threonine
中文名称
——
中文别名
——
英文名称
DL-Threonine
英文别名
threonin;threonine;Thr;2-Azaniumyl-3-hydroxybutanoate
CAS
——
化学式
C4H9NO3
mdl
MFCD00066665
分子量
119.12
InChiKey
AYFVYJQAPQTCCC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-2.9
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:83.6
-
氢给体数:3
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 L-别苏氨酸 (2S,3S)-2-amino-3-hydroxybutanoic acid 144-98-9 C4H9NO3 119.12 L-苏氨酸 L-threonine 72-19-5 C4H9NO3 119.12 —— N-methyl-threonine 855223-97-1 C5H11NO3 133.147 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 D-苏氨酸 D-Threonine 632-20-2 C4H9NO3 119.12 L-苏氨酸 L-threonine 72-19-5 C4H9NO3 119.12 D-别苏氨酸 D-allo-threonine 24830-94-2 C4H9NO3 119.12 L-别苏氨酸 (2S,3S)-2-amino-3-hydroxybutanoic acid 144-98-9 C4H9NO3 119.12 —— L-threonine 72-19-5 C4H9NO3 119.12 —— D-threonine —— C4H9NO3 119.12 —— 2-amino-3-hydroxybutyric acid methyl ester 3373-59-9 C5H11NO3 133.147 —— N,N-dimethythreonine —— C6H13NO3 147.174 —— N,N-dimethyl-L-threonine 2812-36-4 C6H13NO3 147.174 —— ethyl 2-amino-3-hydroxybutanoate 3182-94-3 C6H13NO3 147.174
反应信息
-
作为反应物:描述:DL-Threonine 在 pyridinium polyhydrogen fluoride 、 potassium chloride 、 sodium nitrite 作用下, 反应 72.0h, 以36%的产率得到2-氯-3-羟基-丁酸参考文献:名称:由α-氨基酸制备α-溴代和α-氯代羧酸†摘要:48:52(w / w)氟化氢/吡啶中的α-氨基酸重氮化以及过量的卤化钾导致相应的α-卤代羧酸具有良好或极好的收率(表1和2)。DOI:10.1002/hlca.19830660404
-
作为产物:描述:参考文献:名称:Diastereoselective synthesis of α-amino-β-hydroxyacids摘要:DOI:10.1016/s0040-4039(01)91236-5
-
作为试剂:描述:水合茚三酮 在 DL-Threonine 、 十六烷基三甲基溴化铵 作用下, 以 acetate buffer 为溶剂, 生成 diketohydrindylidenediketohydrindamine参考文献:名称:Kabir-ud-Din; Bano, Manzoora; Khan, Iqrar A., Journal of the Indian Chemical Society, 2004, vol. 81, # 12, p. 1111 - 1118摘要:DOI:
文献信息
-
Peptide ligation by chemoselective aminonitrile coupling in water作者:Pierre Canavelli、Saidul Islam、Matthew W. PownerDOI:10.1038/s41586-019-1371-4日期:2019.7N-to-C peptide ligation. Our model unites prebiotic aminonitrile synthesis and biological α-peptides, suggesting that short N-acyl peptide nitriles were plausible substrates during early evolution.Prebiotic peptide formation is achieved through chemoselective, high-yielding ligation of α-aminonitriles in water, showing selectivity for α-peptide coupling and tolerance of all proteinogenic amino acid residues酰胺键的形成是化学和生物学中最重要的反应之一 1-4,但目前还没有化学方法可以在水中实现 α-肽连接,从而耐受肽连接位点的所有 20 种蛋白质氨基酸。通用遗传密码确立了肽的生物学作用早于生命最后一个普遍的共同祖先,并且肽在生命起源中发挥了重要作用5-9。硫在柠檬酸循环、非核糖体肽合成和聚酮化合物生物合成中的重要作用指向在生命进化过程中,硫酯依赖性肽连接先于 RNA 依赖性蛋白质合成 5,9-13。然而,尚未证明氨酰基硫酯形成的稳健机制。在这里,我们报告了一种化学选择性,高产 α-氨基腈连接,仅利用益生元合理的分子——硫化氢、硫代乙酸盐 12,14 和铁氰化物 12,14-17 或氰基乙炔 8,14——在水中产生 α-肽。这种连接对 α-氨基腈偶联具有极高的选择性,并能耐受所有 20 个蛋白质氨基酸残基。两个基本特征使肽能够在水中连接:α-氨基腈的反应性和 pKaH 使它们与中性 pH 值的
-
[EN] BENZIMIDAZOLE DERIVATIVES AS BROMODOMAIN INHIBITORS<br/>[FR] DÉRIVÉS DE BENZIMIDAZOLE COMME INHIBITEURS DES BROMODOMAINES申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2016146738A1公开(公告)日:2016-09-22Compounds of formula (I) and salts thereof: wherein R1, R2, R3, R4 are defined herein. Compounds of formula (I) and salts thereof have been found to inhibit the binding of the BET family of bromodomain proteins to, for example, acetylated lysine residues and thus may have use in therapy, for example in the treatment of autoimmune and inflammatory diseases, such as rheumatoid arthritis; and cancers.
-
Preparation and characterisation of some mixed ligand complexes of chromium nitrilotriacetate with amino acids作者:C.L. Sharma、P.K. Jain、T.K. DeDOI:10.1016/0022-1902(80)80139-4日期:1980.1The octahedral complexes of the type K[Cr(NTA)(A-A)] and K2[Cr(NTA)(B)2] where NTA = nitrilotriacetate, A-A = deprotonated histidine, proline phenylanthranilic acid, imminodiacetic acid, glutamic acid, aspartic acid, anthranilic acid, threonine, methionine, valine, glycylglycine and glycine and B = deprotonated nicotinic acid, hippuric acid, m and p-aminobenzoic acid, have been prepared and characterised
-
Dehydrodipeptide Hydrogelators Containing Naproxen <i>N</i>-Capped Tryptophan: Self-Assembly, Hydrogel Characterization, and Evaluation as Potential Drug Nanocarriers作者:Helena Vilaça、Ana C. L. Hortelão、Elisabete M. S. Castanheira、Maria-João R. P. Queiroz、Loic Hilliou、Ian W. Hamley、José A. Martins、Paula M. T. FerreiraDOI:10.1021/acs.biomac.5b01006日期:2015.11.9In this work, we introduce dipeptides containing tryptophan N-capped with the nonsteroidal anti-inflammatory drug naproxen and C-terminal dehydroamino acids, dehydrophenylalanine (ΔPhe), dehydroaminobutyric acid (ΔAbu), and dehydroalanine (ΔAla) as efficacious protease resistant hydrogelators. Optimized conditions for gel formation are reported. Transmission electron microscopy experiments revealed that the hydrogels consist of networks of micro/nanosized fibers formed by peptide self-assembly. Fluorescence and circular dichroism spectroscopy indicate that the self-assembly process is driven by stacking interactions of the aromatic groups. The naphthalene groups of the naproxen moieties are highly organized in the fibers through chiral stacking. Rheological experiments demonstrated that the most hydrophobic peptide (containing C-terminal ΔPhe) formed more elastic gels at lower critical gelation concentrations. This gel revealed irreversible breakup, while the C-terminal ΔAbu and ΔAla gels, although less elastic, exhibited structural recovery and partial healing of the elastic properties. A potential antitumor thieno[3,2-b]pyridine derivative was incorporated (noncovalently) into the gel formed by the hydrogelator containing C-terminal ΔPhe residue. Fluorescence and Förster resonance energy transfer measurements indicate that the drug is located in a hydrophobic environment, near/associated with the peptide fibers, establishing this type of hydrogel as a good drug-nanocarrier candidate.在这项工作中,我们介绍了含有色氨酸、N端以非甾体抗炎药萘普生封端、C端为脱水氨基酸(脱水苯丙氨酸(ΔPhe)、脱水氨基丁酸(ΔAbu)和脱水丙氨酸(ΔAla))的二肽,这些二肽作为有效的蛋白酶抵抗型水凝胶形成剂。我们报告了最佳凝胶化条件。透射电子显微镜实验揭示,水凝胶由通过肽自组装形成的微/纳米尺寸纤维网络组成。荧光光谱和圆二色光谱表明,自组装过程是由芳香族基团的堆积相互作用驱动的。萘普生部分的萘环在纤维中通过手性堆积高度有序。流变学实验证明,最疏水的肽(含C端ΔPhe)在较低的临界凝胶化浓度下形成了更具弹性的凝胶。这种凝胶显示出不可逆的破碎,而C端ΔAbu和ΔAla凝胶虽然弹性较差,但表现出结构恢复和部分弹性性能的愈合。将一种潜在的抗肿瘤噻吩并[3,2-b]吡啶衍生物(非共价地)掺入含C端ΔPhe残基的凝胶形成剂形成的水凝胶中。荧光和福斯特共振能量转移测量表明,药物位于疏水环境中,靠近/与肽纤维相连,这表明这种类型的水凝胶是一个良好的药物纳米载体候选者。
-
Useful Reagents for Introduction of Boc and Fmoc Protective Groups to Amines: Boc-DMT and Fmoc-DMT作者:Munetaka Kunishima、Kazuhito Hioki、Mizuho Kinugasa、Michiko Kishimoto、Miho Fujiwara、Shohei TaniDOI:10.1055/s-2006-942389日期:2006.6New amino-protecting reagents, Boc-DMT and Fmoc-DMT, were prepared, and found to be useful for the introduction of Boc and Fmoc groups into amines. Both the reagents can protect various amines including amino acids in good yield in aqueous media. Since the reagents are neither unstable nor irritating, they are practically useful.
表征谱图
-
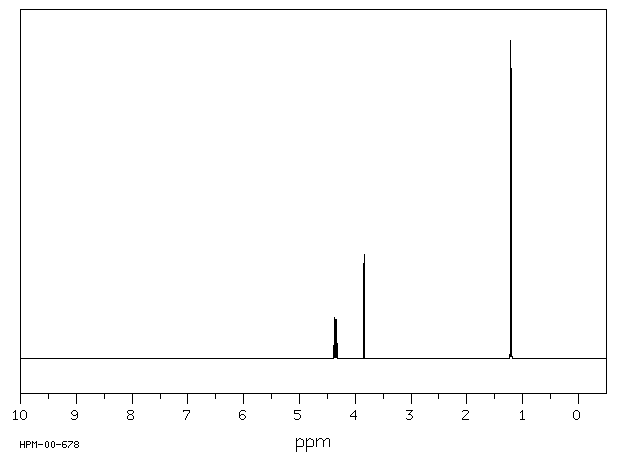
氢谱1HNMR
-
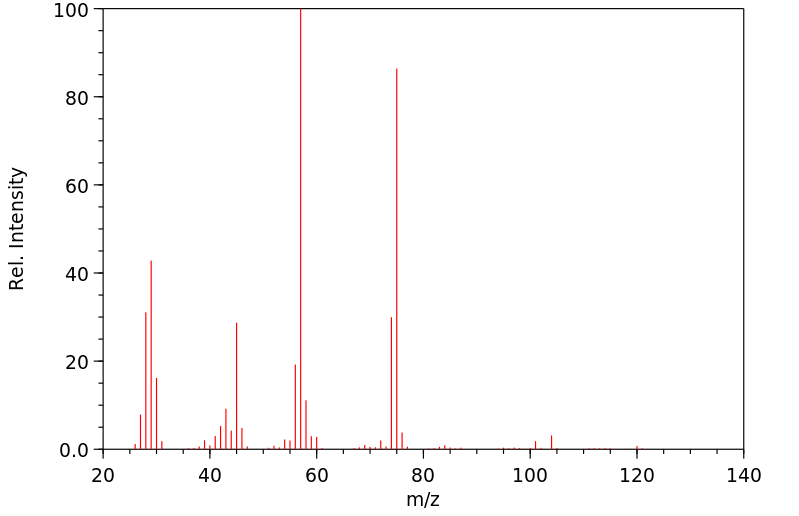
质谱MS
-
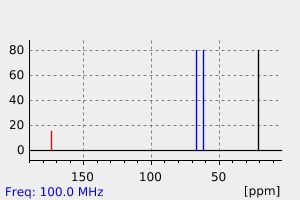
碳谱13CNMR
-
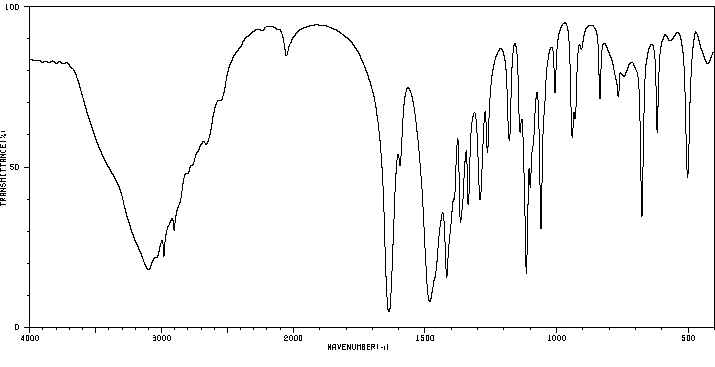
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸