4-亚硝基苯甲醚 | 1516-21-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:23-25 °C
-
沸点:229.2±23.0 °C(Predicted)
-
密度:1.10±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:38.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2909309090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲氧苯胺 4-methoxy-aniline 104-94-9 C7H9NO 123.155 4-亚硝基苯酚 p-nitrosophenol 104-91-6 C6H5NO2 123.111 4-硝基苯甲醚 4-Nitroanisole 100-17-4 C7H7NO3 153.137 —— p-methoxyphenylhydroxylamine 4546-20-7 C7H9NO2 139.154 邻甲氧基苯胺 2-methoxy-phenylamine 90-04-0 C7H9NO 123.155 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-亚硝基苯酚 p-nitrosophenol 104-91-6 C6H5NO2 123.111 甲氧苯胺 4-methoxy-aniline 104-94-9 C7H9NO 123.155 4-硝基苯甲醚 4-Nitroanisole 100-17-4 C7H7NO3 153.137 —— p-methoxyphenylhydroxylamine 4546-20-7 C7H9NO2 139.154 —— 4-nitroso-4'-methoxydiphenylamine 7696-66-4 C13H12N2O2 228.25 对硝基苯酚 4-nitro-phenol 100-02-7 C6H5NO3 139.111 4-甲氧基二苯胺 N-(4-methoxyphenyl)phenylamine 1208-86-2 C13H13NO 199.252 —— Diazene, bis(4-methoxyphenyl)-, (1E)- 501-58-6 C14H14N2O2 242.277 4,4''-二甲氧基氧化偶氮苯 bis(4-methoxyphenyl)diazene 501-58-6 C14H14N2O2 242.277
反应信息
-
作为反应物:参考文献:名称:苯甲醚硝化的机理。摘要:DOI:10.1021/ja01185a036
-
作为产物:描述:参考文献:名称:由亚硝基芳烃和硼酸合成二(杂)芳基胺:一般,轻度和无过渡金属的偶联摘要:据报道,通过亚硝基芳烃和硼酸之间的无过渡金属交叉偶联来合成二(杂)芳基胺。该方法实验上简单,快速,温和且可扩展,并且具有宽泛的官能团耐受性,包括羰基,硝基,卤素,游离的OH和NH基团。它还允许合成位阻化合物。DOI:10.1021/acs.orglett.8b00473
-
作为试剂:描述:参考文献:名称:亚硝基苯交叉二聚:溶液和固态的结构选择性摘要:摘要 亚硝基苯形成二聚分子结构(偶氮二氧化物)的可能性被用作溶液和固态分子间选择性研究的模型。通过变温 1H NMR、固态 NMR (CP MAS)、IR 和 ab initio 计算研究了对和间取代亚硝基苯对的不同组合的交叉二聚。很明显,对亚硝基苯具有非选择性,因为它与所有研究的亚硝基苯伙伴形成二聚体。相反,对甲氧基亚硝基苯在大多数情况下不会形成二聚体。可以通过晶格中分子排列的影响来解释溶液中形成二聚体的能力与固态不同的证据。DOI:10.1016/j.molstruc.2010.05.034
文献信息
-
Synthesis of Azoxybenzenes by Reductive Dimerization of Nitrosobenzene作者:Yu-Feng Chen、Jing Chen、Li-Jen Lin、Gary Jing ChuangDOI:10.1021/acs.joc.7b01887日期:2017.11.3Herein we report an effective and simple preparation method of substituted azoxybenzenes by reductive dimerization of nitrosobenzenes. This procedure requires no additional catalyst/reagent and can be applied to substrates with a wide range of substitution patterns.在本文中,我们报道了通过亚硝基苯的还原二聚作用制备取代的乙氧基苯的有效而简单的方法。该程序不需要额外的催化剂/试剂,并且可以应用于具有广泛取代模式的底物。
-
New nitrite ionic liquid (IL-ONO) and nanoparticles of organosilane-based nitrite ionic liquid immobilized on silica as nitrosonium sources for electrophilic aromatic nitrosation作者:Hassan Valizadeh、Mohammad Amiri、Ashkan ShomaliDOI:10.1016/j.crci.2011.09.012日期:2011.12improved method for the synthesis of nitrosoarenes has been developed using a new nitrite ionic liquid (IL-ONO) and immobilized nitrite ionic liquid. These ionic liquids play as nitrosonium sources for electrophilic aromatic nitrosation of active aromatics at 0–5 °C. Their action was accomplished in water and the satisfactory results were obtained under the mild conditions in short reaction time.
-
An Exceptionally Stable Ti Superoxide Radical Ion: A Novel Heterogeneous Catalyst for the Direct Conversion of Aromatic Primary Amines to Nitro Compounds作者:Gajanan K. Dewkar、Milind D. Nikalje、Iliyas Sayyed Ali、Abhimanyu S. Paraskar、H. S. Jagtap、A. SudalaiDOI:10.1002/1521-3773(20010119)40:2<405::aid-anie405>3.0.co;2-6日期:2001.1.19A matrix-bound superoxide radical anion, generated by treating Ti(OR)4 (R=iPr, nBu) with H2 O2 , is a selective heterogeneous catalyst for the oxidation of anilines to the corresponding nitroarenes with 50 % aqueous H2 O2 [Eq. (1)]. Yields of 82-98 % are obtained, even with anilines bearing electron-withdrawing substituents (R=NO2 , COOH).
-
Transition metal porphyrins as catalysts in the oxidation of nitroso compounds
-
Nonlinear Hammett Relationships in the Reaction of Peroxomonosulfate Anion (HOOSO3-) with meta- and para-Substituted Anilines in Alkaline Medium作者:Subbiah Meenakshisundaram、Ramanathan SockalingamDOI:10.1135/cccc20010897日期:——
The HOOSO3- oxidation of eleven
meta - andpara -substituted anilines to the corresponding nitrosobenzenes at pH ≈ 11 was characterized by the rate equationv =kK [OX][An]/(1 +K [An]). Formation constant of the reactive intermediate and its rate of decomposition were evaluated separately for ascertaining the structure-reactivity relationships. Under the experimental conditions the dianion, -O-O-SO3- is probably the effective electrophile. Kinetic data can be rationalized by a bimolecular process which involves the attack of nucleophilic nitrogen atom on the peroxidic oxygen. The highlight of the study is the opposite curvatures observed in the nonlinear Hammett plots of first-order rate constantk and the "equilibrium" constantK , being concave downward and upward, respectively.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
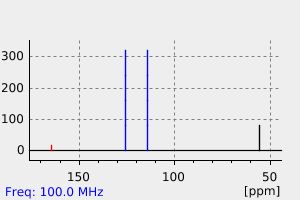
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息