对戊氧基苯酚 | 79-53-8
中文名称
对戊氧基苯酚
中文别名
——
英文名称
chloropentafluoroacetone
英文别名
1-chloro-1,1,3,3,3-pentafluoropropan-2-one
CAS
79-53-8
化学式
C3ClF5O
mdl
MFCD00042218
分子量
182.477
InChiKey
OJUUDWYPNQALHZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-133°C
-
沸点:7,8°C
-
密度:1.6463 (estimate)
-
蒸汽压力:1.42e+03 mmHg
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:10
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:6
安全信息
-
危险等级:GAS, TOXIC
-
危险类别码:R26
-
危险品运输编号:UN 3308
-
安全说明:S26,S36/37/39,S45
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Some properties of polyfluoroacyl- and polyfluoroacetonyl fluorosulfates摘要:DOI:10.1007/bf00956676
-
作为产物:描述:参考文献:名称:Fluoroaliphatic esters of fluorosulfonic acid. 4. Reactions of ?-fluorosulfatoperfluoro ketones with nucleophilic reagents摘要:alpha-Fluorosulfatoperfluoroethyl isopropyl ketone I and fluorosulfatopentafluoroacetone II react with alkali metal chlorides and bromides to form the corresponding alpha-haloperfluoro ketones as a result of direct nucleophilic substitution.DOI:10.1007/bf00958254
-
作为试剂:描述:五氟烯丙基氟硫酸盐 在 磷酸酐 、 potassium fluoride 作用下, 以 二乙二醇二甲醚 、 对戊氧基苯酚 为溶剂, 生成 1-(1,1,1,2,3,3-hexafluoro-3-chloro-2-propoxy)-pentafluoro-2-propene参考文献:名称:Polyfluoroallyloxy compounds, their preparation and copolymers therefrom摘要:聚氟碳酰化合物(如聚氟酮或聚氟羧酸氟化物)与氟离子和聚氟烯基氯化物、溴化物或氟磺酸酯等反应,产生聚氟烯氧基化合物,例如CF.sub.2 .dbd.CFCF.sub.2 OCF.sub.2 CF.sub.2 CN。聚氟烯氧基化合物与四氟乙烯、氯三氟乙烯或偏氟乙烯等乙烯基不饱和单体共聚合,形成可塑性聚合物,在某些情况下具有导电性或可湿润并可染色。公开号:US04273728A1
文献信息
-
Synthesis and thermolysis of highly halogenated Δ<sup>1</sup>-pyrazolines作者:Roger K. Huff、Eric G. SavinsDOI:10.1039/c39800000742日期:——The pyrolysis of highly halogenated Δ1-pyrazolines gives, in addition to cyclopropanes, rearranged olefins.高度卤化的Δ的热解1 -pyrazolines给出,除了环丙烷,重排的烯烃。
-
The synthesis of fluorine-containing pterins作者:Caroline Dunn、Colin L. Gibson、Colin J. SucklingDOI:10.1016/0040-4020(96)00782-x日期:1996.9The synthesis of some 7,7-difluoro-7,8-dihydropterins and pterins with fluoroalkyl subsitutents at the 6 or 7 positions from fluorine-containing aliphatic precursors and suitably substituted pyrimidines is described. The fluorine-containing pterins were found to be very insoluble and also stable to nucleophiles and bases in dilute aqueous solution.
-
The synthesis of perhalogeno-organic borates from perhalogenoketones作者:E. W. Abel、N. Giles、D. J. Walker、J. N. WingfieldDOI:10.1039/j19710001991日期:——Hexafluoroacetone and other polyfluorinated ketones react with boron halides, organoboron halides, and alkylthioboranes to form a wide range of perhalogeno-organic borates and related compounds.
-
Perhalogeno-ketone fissions of Si–N, Ge–N, and Sn–N bonds作者:E. W. Abel、J. P. CrowDOI:10.1039/j19680001361日期:——Hexafluoroacetone, monochloropentafluoroacetone, and dichlorotetrafluoroacetone cause fission of the silicon–nitrogen bond in certain aminosilanes, to form the corresponding insertion products. Hexafluoroacetone is found to insert similarly into the germanium–nitrogen and the tin–nitrogen bond. Insertion into the silicon–nitrogen bonds of a diazasilacyclopentane also causes polymerization.
-
1-Trifluoromethyl-1,2,2-triphenylethylenes. Synthesis and postcoital antifertility activity作者:William J. Middleton、Diana Metzger、Jack A. SnyderDOI:10.1021/jm00294a013日期:1971.12is described, and the postcoital and uterotropic activities of these 1-trifluoromethyl-1,2,2-triphenylethylenes are determined. The parent compound and 3 substituted analogs were prepared by the stepwise replacement of the vinylic F atoms of hexafluoropropene with aryl groups from ArLi reagents. The postcoital antifertility and uterotrophic activities in the rat were determined by treating rats with
表征谱图
-
氢谱1HNMR
-
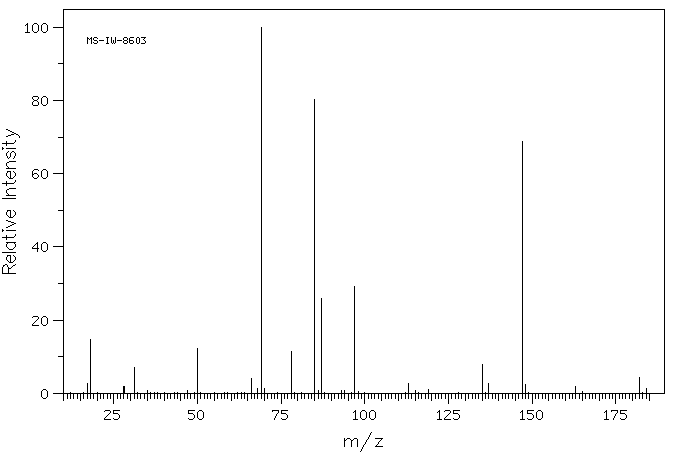
质谱MS
-
碳谱13CNMR
-
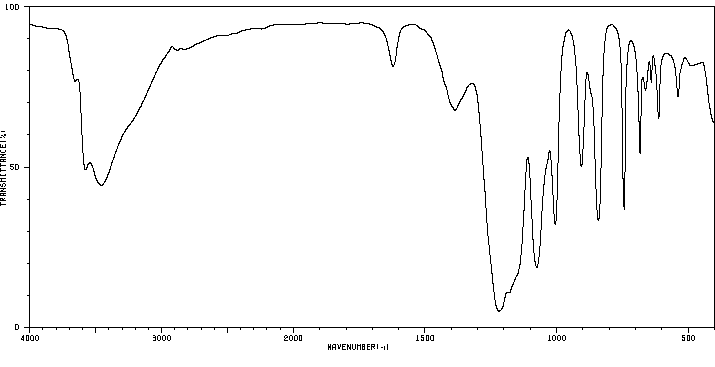
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷