N-(苄氧羰基)-L-丙氨酰-L-丙氨酸 | 16012-70-7
中文名称
N-(苄氧羰基)-L-丙氨酰-L-丙氨酸
中文别名
苄氧羰基-L-丙氨酰-L-丙氨酸
英文名称
benzyloxycarbonyl-L-alanyl-L-alanine
英文别名
Z-Ala-Ala-OH;Cbz-L-Ala-L-Ala-OH;N-[(benzyloxy)carbonyl]-L-alanyl-L-alanine;N-Cbz-L-Ala-L-Ala-OH;Cbz-Ala-Ala;Z-L-Ala-L-Ala-OH;(2S)-2-[[(2S)-2-(phenylmethoxycarbonylamino)propanoyl]amino]propanoic acid
CAS
16012-70-7
化学式
C14H18N2O5
mdl
——
分子量
294.307
InChiKey
JBGCVTHXXTVYIP-UWVGGRQHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:136-140 °C
-
沸点:573.0±45.0 °C(Predicted)
-
密度:1.252±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:21
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:105
-
氢给体数:3
-
氢受体数:5
安全信息
-
海关编码:2924299090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:|-20°C|
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Z-Ala-Ala-OH
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Z-Ala-Ala-OH
CAS number: 16012-70-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C14H18N2O5
Molecular weight: 294.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Z-Ala-Ala-OH
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Z-Ala-Ala-OH
CAS number: 16012-70-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C14H18N2O5
Molecular weight: 294.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲基N-[(苄氧基)羰基]-L-丙氨酰-L-丙氨酸酯 N-[(benzyloxy)carbonyl]-L-alanyl-L-alanine methyl ester 2483-51-4 C15H20N2O5 308.334 —— N-benzyloxycarbonyl-L-alanyl-L-alanine ethyl ester 5673-69-8 C16H22N2O5 322.361 —— N-benzyloxycarbonyl-alanyl-alanyl-leucine 54865-22-4 C20H29N3O6 407.467 —— Z-Ala-Ala-Arg-OH 50465-92-4 C20H30N6O6 450.495 苄氧羰基-L-丙氨酸 N-Cbz-Ala 1142-20-7 C11H13NO4 223.229 N-CBZ-DL-丙氨酸 N-benzyloxycarbonylalanine 4132-86-9 C11H13NO4 223.229 Z-L-丙氨酸甲酯 (S)-2-benzyloxycarbonylamino-propionic acid methyl ester 28819-05-8 C12H15NO4 237.255 —— N-benzyloxycarbonyl-L-alanyl chloride 49760-60-3 C11H12ClNO3 241.674 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Z-Ala-Ala-Ala 13585-98-3 C17H23N3O6 365.386 甲基N-[(苄氧基)羰基]-L-丙氨酰-L-丙氨酸酯 N-[(benzyloxy)carbonyl]-L-alanyl-L-alanine methyl ester 2483-51-4 C15H20N2O5 308.334 —— benzyl ((S)-1-(((S)-1-hydroxypropan-2-yl)amino)-1-oxopropan-2-yl)carbamate 129221-00-7 C14H20N2O4 280.324 —— N-benzyloxycarbonyl-L-alanyl-L-alanine ethyl ester 5673-69-8 C16H22N2O5 322.361 —— N-(N-benzyloxycarbonyl-alanyl)-alanine amide 50444-54-7 C14H19N3O4 293.323 —— (2S)-2-[N-(Benzyloxycarbonyl-(S)-alanyl-(S)-alanyl)amino]-4-methylpentan-1-ol 177657-10-2 C20H31N3O5 393.483 —— N-[(benzyloxy)carbonyl]-L-alanyl-L-alanyl-L-asparagine —— C18H24N4O7 408.411 —— Z-Ala-Ala-Leual 177657-11-3 C20H29N3O5 391.467 —— benzyl N-[(2S)-1-oxo-1-[[(2S)-1-oxo-1-(2-phenylethylamino)propan-2-yl]amino]propan-2-yl]carbamate 84899-49-0 C22H27N3O4 397.474 —— Z-Ala-Ala-Leu-NH2 76264-64-7 C20H30N4O5 406.482 —— Z-Ala-Ala-Pheol 178910-89-9 C23H29N3O5 427.5 —— Z-Ala-Ala-Phe-OH 19245-91-1 C23H27N3O6 441.484 —— Z-Ala-Ala-Pro-OH 61430-05-5 C19H25N3O6 391.424 —— rac-5,7-Bis-(Z-(L)-alanyl-(L)-alanine amidyl)undecan-6-ol —— C39H58N6O9 754.924 —— rac-6,8-Bis-(Z-(L)-alanyl-(L)-alanine amidyl)tridecan-7-ol —— C41H62N6O9 782.978 N-Cbz-L-丙氨酰-L-丙氨酸琥珀酰亚胺酯 N-Cbz-L-alanyl-L-alanine succinimido ester 16946-96-6 C18H21N3O7 391.381 —— Z-L-Ala-ψ(CSNH)-L-Ala-OMe 87043-02-5 C15H20N2O4S 324.401 —— Benzyloxycarbonylalanyl-alanyl-proline Ethylamide 84914-63-6 C21H30N4O5 418.493 苄氧羰基-L-丙氨酸 N-Cbz-Ala 1142-20-7 C11H13NO4 223.229 —— erythro Cbz-alanyl-alanyl-phenylalanyl epoxide —— C24H29N3O5 439.511 —— threo Cbz-alanyl-alanyl-phenylalanyl epoxide —— C24H29N3O5 439.511 —— Cbz-alanyl-alanyl-phenylalanyl bromomethane 159141-68-1 C24H28BrN3O5 518.407 —— ethyl ((benzyloxy)carbonyl)-L-alaninate 60625-90-3 C13H17NO4 251.282 丙氨酰丙氨酰丙氨酸 L-ala-L-ala-L-ala 5874-90-8 C9H17N3O4 231.252 —— 1-(N-carbobenzyloxy-L-alanyl-L-alanyl-L-phenylalanyl)-2-chloroacetyl hydrazine 142182-09-0 C25H30ClN5O6 531.996 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Losse, Guenter; Stiehl, Hans-Ulrich, Zeitschrift fur Chemie, 1981, vol. 21, # 5, p. 188摘要:DOI:
-
作为产物:描述:苄氧羰基-L-丙氨酸 在 sodium hydroxide 、 三乙胺 、 N,N'-二环己基碳二亚胺 作用下, 以 甲醇 、 二氯甲烷 为溶剂, 反应 24.0h, 生成 N-(苄氧羰基)-L-丙氨酰-L-丙氨酸参考文献:名称:真菌代谢物。IV。从多孢木霉菌中合成抗生素肽曲霉菌素BV。摘要:从多木霉菌中分离出的抗生素二十碳三肽孢霉菌素BV是通过N,N'-二环己基碳二亚胺法组装五个肽片段而合成的。合成的肽与天然肽相同。DOI:10.1248/cpb.38.2997
文献信息
-
Synthesis and Structure−Activity Relationships of 2-Pyridones: A Novel Series of Potent DNA Gyrase Inhibitors as Antibacterial Agents作者:Qun Li、Daniel T. W. Chu、Akiyo Claiborne、Curt S. Cooper、Cheuk M. Lee、Kathleen Raye、Kristine B. Berst、Pamela Donner、Weibo Wang、Lisa Hasvold、Anthony Fung、Zhenkun Ma、Michael Tufano、Robert Flamm、Linus L. Shen、John Baranowski、Angela Nilius、Jeff Alder、Jonathan Meulbroek、Kennan Marsh、DeAnne Crowell、Yuhua Hui、Louis Seif、Laura M. Melcher、Rodger Henry、Steven Spanton、Ramin Faghih、Larry L. Klein、S. Ken Tanaka、Jacob J. PlattnerDOI:10.1021/jm960207w日期:1996.1.1Two novel series of 2-pyridones were synthesized by transposition of the nitrogen of 4-quinolones to the bridgehead position. This subtle interchange of the nitrogen atom with a carbon atom yielded two novel heterocyclic nuclei, pyrido[1,2-alpha]pyrimidine and quinolizine, which had not previously been evaluated as antibacterial agents and were found to be potent inhibitors of DNA gyrase. Quinolizines通过将4-喹诺酮类的氮转位至桥头位置,合成了两个新颖的2-吡啶酮系列。氮原子与碳原子的这种微妙的交换产生了两个新的杂环核,吡啶并[1,2-α]嘧啶和喹啉嗪,它们先前未被评估为抗菌剂,并且被发现是DNA促旋酶的有效抑制剂。(S)-45a(ABT-719)等在9位甲基的喹唑啉酮具有出色的广谱抗菌活性。最值得注意的是,它们对耐药菌具有活性,例如耐甲氧西林的金黄色葡萄球菌,耐万古霉素的肠球菌菌株和耐环丙沙星的生物。此外,2-吡啶酮还具有良好的理化和药代动力学特性。这些2-吡啶酮是通过10-17个线性转化从商购可得的起始原料合成的。通过X射线晶体学分析确定由该序列产生的加合物(S)-45a(ABT-719)的结构。
-
Synthesis of N-urethane Protected β-Amino Alcohols Employing <i>N</i>-(protected-α-aminoacyl)benzotriazoles作者:Vommina V. Sureshbabu、N.S. Sudarshan、L. Muralidhar、N. NarendraDOI:10.3184/030823407x272985日期:2007.12
A simple and racemisation-free synthesis of N-urethane protected α-amino/peptidyl alcohols by the reduction of the corresponding easily accessible N-acylbenzotriazoles is described. The method is practical, straightforward, fast and efficient for the synthesis of amino/peptidyl alcohols. All the alcohols made were isolated in high yields and purity.
-
N-benzhydryl-glycolamide esters (OBg esters) as carboxyl protecting groups in pept1de synthesis作者:Muriel Amblard、Marc Rodriguez、Jean MartinezDOI:10.1016/s0040-4020(01)86014-2日期:1988.1N-Benzhydryl-glycolamide esters (OBg esters) of various N-protected amino acids have been synthesized. In order to demonstrate their usefulness in peptide chemistry, the syntheses of For-Met-Leu-Phe-OH (chemiotactic peptide) and Pro-Leu-Gly-NH2 (MIF) have been carried out. OBg esters are compatible with commonly used protecting groups and are cleanly and selectively removed in mild alkaline conditions
-
Small peptides as modular catalysts for the direct asymmetric aldol reaction: ancient peptides with aldolase enzyme activity作者:Weibiao Zou、Ismail Ibrahem、Pawel Dziedzic、Henrik Sundén、Armando CórdovaDOI:10.1039/b509920j日期:——Simple peptides and their analogues having a primary amino group as the catalytic residue mediate the direct asymmetric intermolecular aldol reaction with high stereoselectivity and furnish the corresponding aldol products with up to 99% ee; this intrinsic ability of highly modular peptides may explain the initial molecular evolution of aldolase enzymes.
-
Elektrochemische Decarboxylierung vonL.-Threonin- und Oligopeptid-Derivaten unter Bildung vonN-Acyl-N, O-acetalen: Herstellung von Oligopeptiden mit Carboxamid-oder Phosphonat-C-Terminus作者:Dieter Seebach、Roland Charczuk、Christian Gerber、Philippe Renaud、Heinz Berner、Helmut SchneiderDOI:10.1002/hlca.19890720302日期:1989.5.3Electrochemical Decarboxylation of L-Threonine and Oligopeptide Derivatives with Formation of N-Acyl-N, O-acetals: Preparation of Oligopeptides with Amide or Phophonate C-Terminus
表征谱图
-
氢谱1HNMR
-
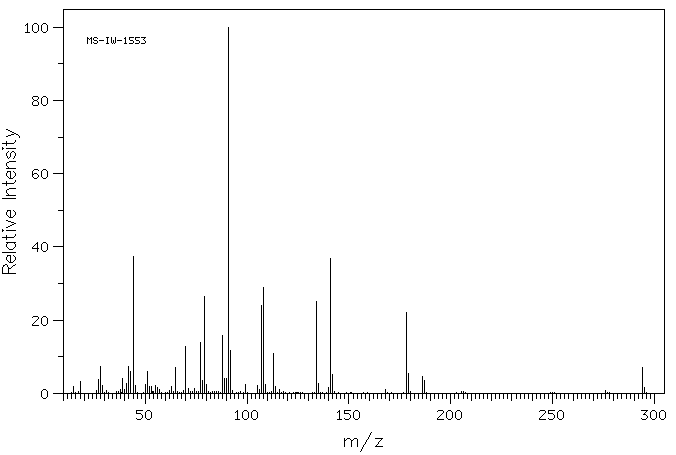
质谱MS
-
碳谱13CNMR
-
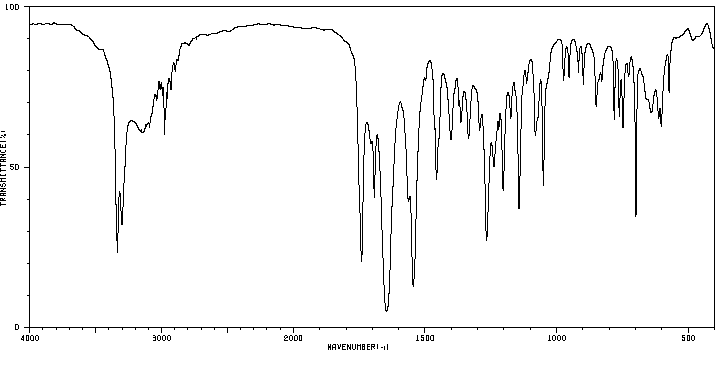
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸