4-(三氟甲基)二苯甲酮 | 728-86-9
中文名称
4-(三氟甲基)二苯甲酮
中文别名
4-(三氟甲基)苯甲酮;4-(三氟甲基)苯甲酮;4-(三氟甲基)苯并苯基酮;4-(三氟甲基)苯并苯基酮, 98+%;4-(三氟甲基)苯甲酮;对三氟甲基苯甲酮
英文名称
phenyl[4-(trifluoromethyl)phenyl]methanone
英文别名
4-trifluoromethylbenzophenone;4-Trifluoromethylbenzophenon;4-(Trifluoromethyl)benzophenone;phenyl-[4-(trifluoromethyl)phenyl]methanone
CAS
728-86-9
化学式
C14H9F3O
mdl
MFCD00000400
分子量
250.22
InChiKey
OHTYZZYAMUVKQS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:114-116 °C(lit.)
-
沸点:309.2±42.0 °C(Predicted)
-
密度:1.2778 (estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:18
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914700090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将容器密封,然后存放在紧密封装的容器中,并储存在阴凉、干燥的地方。
SDS
| Name: | 4-(Trifluoromethyl)Benzophenone 97% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 728-86-9 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 728-86-9 | 4-(Trifluoromethyl)Benzophenone | 97 | 211-974-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 728-86-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 116.00 - 118.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H9F3O
Molecular Weight: 250.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 728-86-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-(Trifluoromethyl)Benzophenone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 728-86-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 728-86-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 728-86-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
4-(三氟甲基)苯甲酮可以作为有机合成中间体和医药中间体,主要应用于实验室研发及化工生产过程。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-苄基-4-三氟甲基苯 1-benzyl-4-(trifluoromethyl)benzene 34239-04-8 C14H11F3 236.237 —— 2-bromo-4'-(trifluoromethyl)benzophenone 222040-98-4 C14H8BrF3O 329.116 3,5-双(三氟甲基)苯甲酮 (3,5-bis(trifluoromethyl)phenyl)(phenyl)methanone 21221-93-2 C15H8F6O 318.218 对三氟甲基苯甲醛 4-Trifluoromethylbenzaldehyde 455-19-6 C8H5F3O 174.122 苯基-[4-(三氯甲基)苯基]甲酮 4-Trichlormethyl-benzophenon 1218-90-2 C14H9Cl3O 299.584 1-(1-苯基乙烯基)-4-(三氟甲基)苯 1-phenyl-1-(4-trifluoromethylphenyl)ethylene 345-88-0 C15H11F3 248.248 4-三氟甲基-二苯甲醇 phenyl(4-(trifluoromethyl)phenyl)methanol 395-23-3 C14H11F3O 252.236 —— phenyl(4-(trifluoromethyl)phenyl)methanamine —— C14H12F3N 251.251 4-(三氟甲基)苯甲酰氟 4-trifluoromethyl-benzoyl fluoride 368-94-5 C8H4F4O 192.113 4-氨基二苯甲酮 (4-aminophenyl)(phenyl)methanone 1137-41-3 C13H11NO 197.236 —— 4-trifluoromethylthiobenzoic acid 1514-09-6 C8H5F3OS 206.188 4-三氟甲基苯甲酰氯 4-trifluoromethyl-phenyl acetyl chloride 329-15-7 C8H4ClF3O 208.567 4-(三氟甲基)苯甲酰胺 p-trifluoromethylbenzamide 1891-90-3 C8H6F3NO 189.137 4-三氟甲基苯甲酸 4-trifluoromethylbenzoic acid 455-24-3 C8H5F3O2 190.122 4-羟基-二苯甲酮 4-Hydroxybenzophenone 1137-42-4 C13H10O2 198.221 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苄基-4-三氟甲基苯 1-benzyl-4-(trifluoromethyl)benzene 34239-04-8 C14H11F3 236.237 4-甲基二苯甲酮 4-Methylbenzophenone 134-84-9 C14H12O 196.249 —— (2-hydroxyphenyl)(4-(trifluoromethyl)phenyl)methanone 34450-48-1 C14H9F3O2 266.219 1-(氯(苯基)甲基)-4-(三氟甲基)-苯 1-(chloro(phenyl)methyl)-4-(trifluoromethyl)-benzene 787-49-5 C14H10ClF3 270.682 1-(1-苯基乙烯基)-4-(三氟甲基)苯 1-phenyl-1-(4-trifluoromethylphenyl)ethylene 345-88-0 C15H11F3 248.248 —— (phenyl)(4-trifluoromethylphenyl)methyl bromide 171628-30-1 C14H10BrF3 315.133 4-三氟甲基-二苯甲醇 phenyl(4-(trifluoromethyl)phenyl)methanol 395-23-3 C14H11F3O 252.236 —— (R)-phenyl(4-(trifluoromethyl)phenyl)methanol —— C14H11F3O 252.236 —— (S)-phenyl(4-(trifluoromethyl)phenyl)methanol 53756-61-9 C14H11F3O 252.236 —— phenyl(4-(trifluoromethyl)phenyl)methanamine —— C14H12F3N 251.251 1-[重氮(苯基)甲基]-4-(三氟甲基)苯 1-(diazo(phenyl)methyl)-4-(trifluoromethyl)benzene 60664-82-6 C14H9F3N2 262.234 —— 1-(2-bromo-1-phenylvinyl)-4-(trifluoromethyl)benzene 365-09-3 C15H10BrF3 327.144 —— 4-(Trifluormethyl)-benzophenon-hydrazon 60664-70-2 C14H11F3N2 264.25 4-三氟甲基苯甲酸 4-trifluoromethylbenzoic acid 455-24-3 C8H5F3O2 190.122 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:环取代对1,1-二芳基烷氧基自由基的O-叶肉重排的影响。产品和时间分辨动力学研究摘要:已经进行了环取代对1,1-二芳基烷氧基自由基反应性影响的产物和时间分辨动力学研究。所述基团经历O-新叶酸移位,以给出异构的1-芳基-1-芳氧基烷基,从其形成相应的芳族酮。重排速率常数受环取代的影响,在吸电子取代基的存在下增加,而在供电子取代基的存在下降低。根据产物和动力学研究的结果,获得了以下迁移能力:4-三氟甲基苯基>苯基≅4-甲基苯基> 4-甲氧基苯基。σ +之间的出色Hammett型相关性获得了取代基常数以及可见吸收带最大值和重排速率常数。实验结果表明,重排受起始的1,1-二芳基烷氧基自由基的电子效应支配,而重排的以碳为中心的自由基的稳定性起次要作用,与类似反应物的过渡态一致,强烈支持了1,1-二芳基烷氧基基团的O-新叶重排通过协调机制进行的假说。DOI:10.1021/jo0502448
-
作为产物:参考文献:名称:通过芳基CO键活化,镍介导的苯酚衍生物的三氟甲基化。摘要:三氟甲基芳烃的药物重要性的提高刺激了更有效的三氟甲基化反应的发展。大量努力集中于铜和钯介导的/催化的芳基卤化物的三氟甲基化。相反,不存在将广泛使用的惰性亲电子试剂如苯酚衍生物转化为相应的三氟甲基化芳烃的通用方法。本文报道的是苯酚衍生物与容易获得的三甲基(三氟甲基)硅烷(TMSCF 3)进行的镍介导的三氟甲基化反应。该策略依赖于PMe 3促进的氧化加成和过渡金属化以及CCl 3CN诱导的还原消除。通过将三氟甲基直接掺入芳族和杂芳族体系(包括生物相关化合物)中,已证明了这种转化的广泛用途。DOI:10.1002/anie.202004116
-
作为试剂:描述:参考文献:名称:在叔丁胺存在下二苯甲酮与乙腈对苯甲酮光敏的新型氰甲基化反应。摘要:DOI:10.1021/jo960648n
文献信息
-
Suzuki–Miyaura Cross-Coupling of <i>N</i>-Acylpyrroles and Pyrazoles: Planar, Electronically Activated Amides in Catalytic N–C Cleavage作者:Guangrong Meng、Roman Szostak、Michal SzostakDOI:10.1021/acs.orglett.7b01575日期:2017.7.7The formation of C–C bonds from amides by catalytic activation of the amide bond has been thus far possible by steric distortion. Herein, we report the first example of a general Pd-catalyzed Suzuki–Miyaura cross-coupling of planar amides enabled by the combination of (i) electronic-activation of the amide nitrogen in N-acylpyrroles and pyrazoles and (ii) the use of a versatile Pd-NHC catalysis platform
-
Nickel-Catalyzed Diaryl Ketone Synthesis by N–C Cleavage: Direct Negishi Cross-Coupling of Primary Amides by Site-Selective <i>N</i>,<i>N</i>-Di-Boc Activation作者:Shicheng Shi、Michal SzostakDOI:10.1021/acs.orglett.6b02952日期:2016.11.18the first amide cross-coupling by direct metal insertion of simple and readily available primary amides. The overall strategy by N,N-di-Boc activation/metal insertion is suitable for a broad range of coupling protocols via acylmetals. Mechanistic experiments suggest high reactivity of N,N-di-Boc activated 1° amides in direct amide C–N cross-couplings.
-
General Method for the Suzuki–Miyaura Cross-Coupling of Primary Amide-Derived Electrophiles Enabled by [Pd(NHC)(cin)Cl] at Room Temperature作者:Peng Lei、Guangrong Meng、Yun Ling、Jie An、Steven P. Nolan、Michal SzostakDOI:10.1021/acs.orglett.7b03191日期:2017.12.15temperature Suzuki–Miyaura cross-coupling of commonly encountered primary benzamides is reported. A combination of site-selective N,N-di-Boc-activation (tert-butoxycarbonyl activation) of the amide nitrogen with practical air- and moisture-stable, well-defined, and highly reactive [Pd(NHC)(cin)Cl] (NHC = N-heterocyclic carbene; cin = cinnamyl) provides a highly effective route to biaryl ketones from primary据报道,室温下,通常遇到的主要苯甲酰胺的Suzuki-Miyaura交叉偶联是一种通用的,高度选择性的方法。酰胺氮的位点选择性N,N -di-Boc活化(叔丁氧基羰基活化)与稳定的空气和水分稳定的,定义明确的和高反应性的[Pd(NHC)(cin)Cl ](NHC = N-杂环卡宾; cin =肉桂基)提供了一种从伯酰胺高收率制备联芳基酮的高效途径。酰胺酰基交叉偶联首次获得了> 1000的TON。
-
Facile Protocol for Catalytic Frustrated Lewis Pair Hydrogenation and Reductive Deoxygenation of Ketones and Aldehydes作者:Tayseer Mahdi、Douglas W. StephanDOI:10.1002/anie.201503087日期:2015.7.13A series of ketones and aldehydes are reduced in toluene under H2 in the presence of 5 mol % B(C6F5)3 and either cyclodextrin or molecular sieves affording a facile metal‐free protocol for reduction to alcohols. Similar treatment of aryl ketones resulted in metal‐free deoxygenation yielding aromatic hydrocarbons.
-
Conversion of Aromatic Amino into Trifluoromethyl Groups through a Sandmeyer-Type Transformation作者:Jianbo Wang、Xi Wang、Yan Xu、Yujing Zhou、Yan ZhangDOI:10.1055/s-0033-1338659日期:——strategy for aromatic trifluoromethylation by converting amino into trifluoromethyl groups via a Sandmeyer-type reaction is reported. The transformation involves diazotization of the aromatic amines with tert-butyl nitrite and hydrochloric acid to form aryldiazonium chlorides, followed by trifluoromethylation with trifluoromethylsilver at low temperature. Various readily available aromatic amines are
表征谱图
-
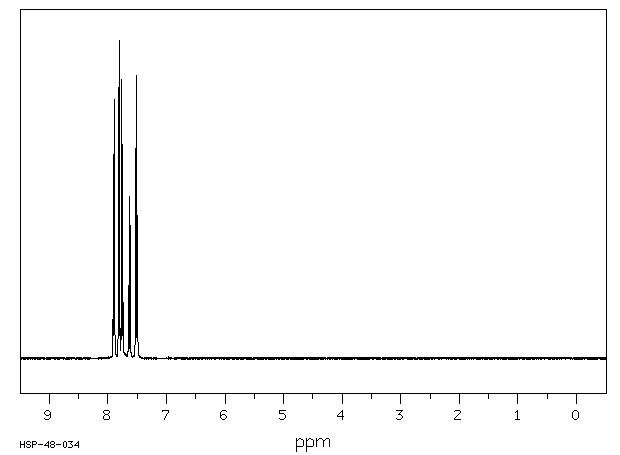
氢谱1HNMR
-
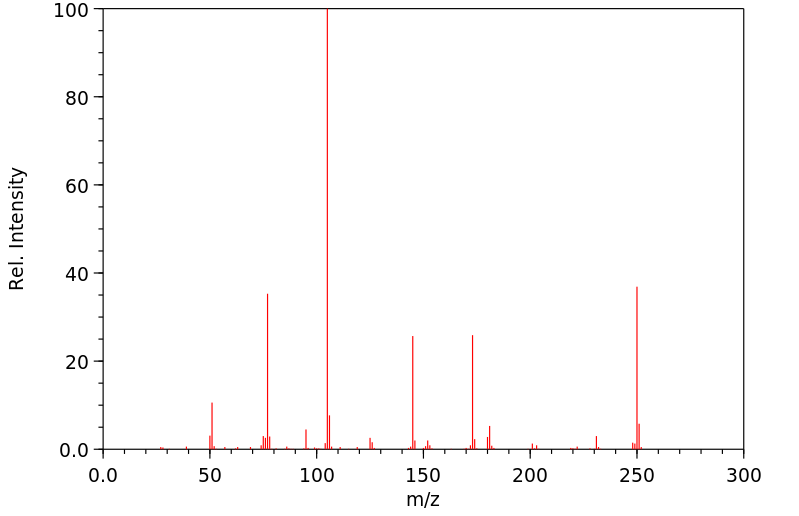
质谱MS
-
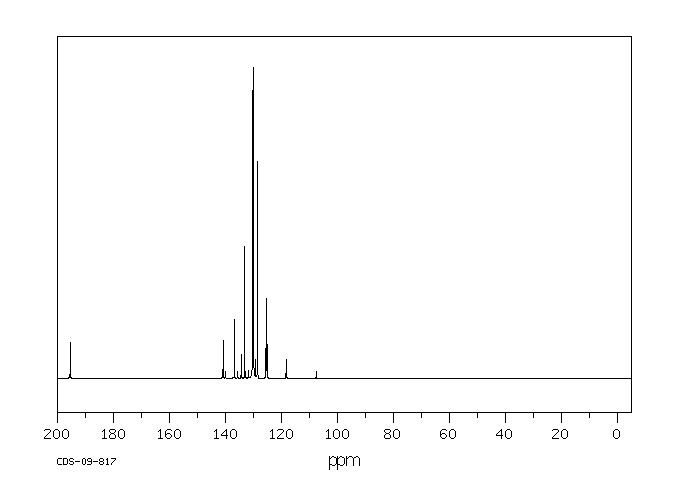
碳谱13CNMR
-
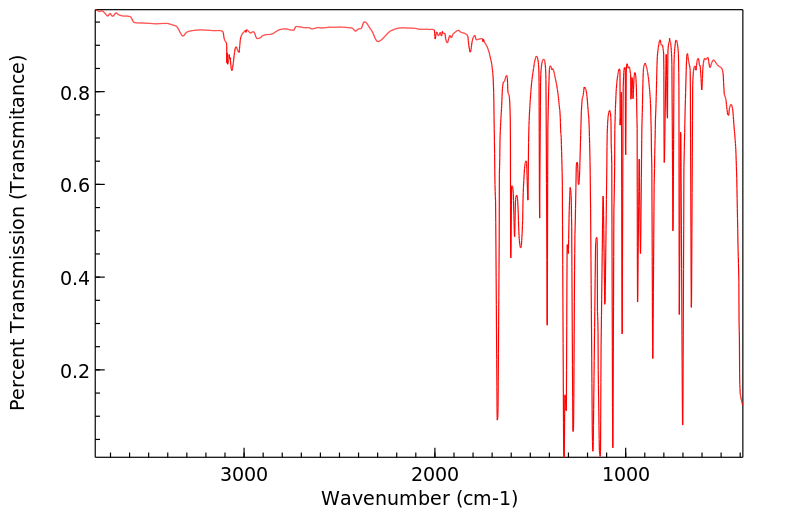
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫