8-十六炔 | 19781-86-3
中文名称
8-十六炔
中文别名
——
英文名称
hexadec-8-yne
英文别名
8-hexadecyne
CAS
19781-86-3
化学式
C16H30
mdl
MFCD00041664
分子量
222.414
InChiKey
YSDKMBBHKZXPME-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:17.51°C (estimate)
-
沸点:115-6°C 0,5mm
-
密度:0.80
计算性质
-
辛醇/水分配系数(LogP):7.7
-
重原子数:16
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
-
储存条件:| 室温 干燥 |
SDS
8-Hexadecyne Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 8-Hexadecyne
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Aspiration hazard Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements
May be fatal if swallowed and enters airways
Precautionary statements:
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
induce vomiting.
[Storage] Store locked up.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 8-Hexadecyne
Percent: >99.0%(GC)
CAS Number: 19781-86-3
Chemical Formula: C16H30
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
8-Hexadecyne
Section 4. FIRST AID MEASURES
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, carbon dioxide.
Suitable extinguishing
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Very pale yellow
Colour:
8-Hexadecyne
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.80
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
8-Hexadecyne
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 8-Hexadecyne
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Aspiration hazard Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements
May be fatal if swallowed and enters airways
Precautionary statements:
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
induce vomiting.
[Storage] Store locked up.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 8-Hexadecyne
Percent: >99.0%(GC)
CAS Number: 19781-86-3
Chemical Formula: C16H30
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
8-Hexadecyne
Section 4. FIRST AID MEASURES
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, carbon dioxide.
Suitable extinguishing
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Very pale yellow
Colour:
8-Hexadecyne
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.80
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
8-Hexadecyne
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:8-十六炔 在 tris-(dibenzylideneacetone)dipalladium(0) 、 三环己基膦 、 甲酸 作用下, 以 1,4-二氧六环 为溶剂, 反应 0.25h, 以90%的产率得到正十六烷参考文献:名称:用羧酸和第 10 族过渡金属配合物对炔烃进行简便的区域和立体选择性加氢金属化:用甲酸对炔烃进行选择性加氢摘要:通过炔烃、羧酸和零价族 10 过渡金属络合物 M(PEt(3))(4) (M =镍、钯、铂)。一项机理研究表明,氢金属化不是通过炔烃与由羧酸与 Pt(PEt(3))(4) 质子化产生的氢化金属反应进行的,而是通过炔烃配位金属络合物与酸。这一发现阐明了长期以来提出的反应机制,该机制通过生成烯基钯中间体和随后在由 Brφnsted 酸和 Pd(0) 配合物的组合催化的各种反应中转化该配合物来进行。DOI:10.1021/ja2069246
-
作为产物:参考文献:名称:Synthesis of 1,2-dialkylcyclopropenes, methyl malvalate, and sterculate摘要:DOI:10.1021/jo00986a012
-
作为试剂:描述:3,5-diphenyl-1-tosyl-2,3-dihydro-1H-pyrrole 在 silver hexafluoroantimonate 、 copper(II) acetate monohydrate 、 bis[dichloro(pentamethylcyclopentadienyl)ruthenium(III)] 、 8-十六炔 、 水 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 12.0h, 生成 4-methyl-N-(4-oxo-2,4-diphenylbutyl)benzenesulfonamide参考文献:名称:铑(III)催化[3 + 2]通过C(sp2)与内部炔烃一起生成5-芳基-2,3-二氢-1H-吡咯的环状结构?H /烯烃功能化摘要:这项研究描述了一种新的铑(III)催化的[3- + 2] 5-芳基-2,3-二氢-1H-吡咯与内部炔烃的反应,该反应使用Cu(OAc)2氧化剂构建螺环系统,包括芳基C(SP的官能化2) H成烯烃CC键的键和加法/质子分解。该方法适用于广泛的5-芳基-2,3-二氢-1H-吡咯和内部炔烃,并能以良好的收率合成螺[indene-1,2'-吡咯烷]结构,并获得优异的收率。区域选择性。DOI:10.1002/anie.201407175
文献信息
-
PROCESS FOR PRODUCTION OF FUSED RING COMPOUND申请人:Miura Masahiro公开号:US20090156832A1公开(公告)日:2009-06-18It is an object of the present invention to provide a method for manufacturing a fused ring compound, with which a fused ring compound that has excellent charge transport property and that has excellent solubility in solvents can be obtained efficiently. The method of the present invention for manufacturing a fused ring compound involves reacting a compound expressed by the following General Formula (1a) and a compound expressed by the following General Formula (1b) in the presence of an amine and a metal complex catalyst: (where Ar 11 and Ar 12 are each independently an atom group constituting an aromatic ring or a heterocyclic ring; X 11 and X 12 are each independently a hydrogen atom or a halogen atom, and at least one is a halogen atom; and R 11 and R 12 are each independently a hydrogen atom, an all group, an alkoxy group, an alkylthio group, an alkylamino group, an alkoxycarbonyl group, an aryl group, a heterocyclic group, or a cyano group, provided that at least one of R 11 and R 12 is not a hydrogen atom).
-
Syngas‐Free Highly Regioselective Rhodium‐Catalyzed Transfer Hydroformylation of Alkynes to α,β‐Unsaturated Aldehydes作者:Guangying Tan、Yimin Wu、Yang Shi、Jingsong YouDOI:10.1002/anie.201902553日期:2019.5.27fundamental and important reaction in both academic research and industry. Conventional methods focus on the conversion of alkynes, CO, and H2 into α,β‐unsaturated aldehydes, but they often suffer from problems associated with operation, regioselectivity, and chemoselectivity. Herein, we disclose an operationally simple, mild, and syngas‐free rhodium‐catalyzed reaction for the hydroformylation of alkynes
-
Iodomesitylene-Catalyzed Oxidative Cleavage of Carbon−Carbon Double and Triple Bonds Using <i>m</i>-Chloroperbenzoic Acid as a Terminal Oxidant作者:Kazunori Miyamoto、Yoshihisa Sei、Kentaro Yamaguchi、Masahito OchiaiDOI:10.1021/ja808829t日期:2009.2.4Transition metal-catalyzed oxidative cleavage of carbon-carbon multiple bonds has emerged as a powerful tool in organic synthesis. High-valent oxometals, mostly of Ru, Os, Mn, Mo, W, and Re, were used catalytically as reactive oxygen transfer agents to the multiple bonds. Reported here for the first time are the organocatalytic versions of the oxidative cleavage reactions. Our method involves use of
-
Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source作者:Yong Ho Lee、Elliott H. Denton、Bill MorandiDOI:10.1038/s41557-020-00621-x日期:2021.2Hydroformylation, a reaction that installs both a C–H bond and an aldehyde group across an unsaturated substrate, is one of the most important catalytic reactions in both industry and academia. Given the synthetic importance of creating new C–C bonds, the development of carboformylation reactions, wherein a new C–C bond is formed instead of a C–H bond, would bear enormous synthetic potential to rapidly
-
Synthesis of Alkylated Benzo[2,1-<i>b</i>:3,4-<i>b</i>′]dithiophenes by Annulative Coupling and Their Direct Arylation under Palladium Catalysis作者:Hiroyuki Watanabe、Jun Kumagai、Hayato Tsurugi、Tetsuya Satoh、Masahiro MiuraDOI:10.1246/cl.2007.1336日期:2007.11.5The annulative coupling of 3,3′-diiodo-2,2′-bithiophene with internal alkynes efficiently proceeds in the presence of a palladium catalyst to afford the corresponding benzo[2,1-b:3,4-b′]dithiophene derivatives. The dithiophenes also undergo palladium-catalyzed direct arylation with aryl bromides at the 2- and 7-positions selectively.
表征谱图
-
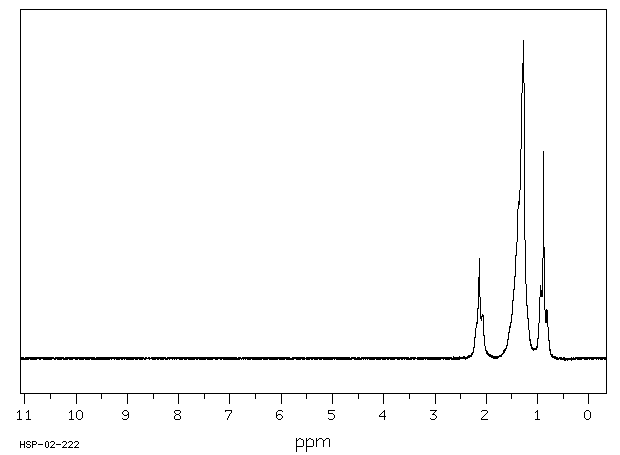
氢谱1HNMR
-
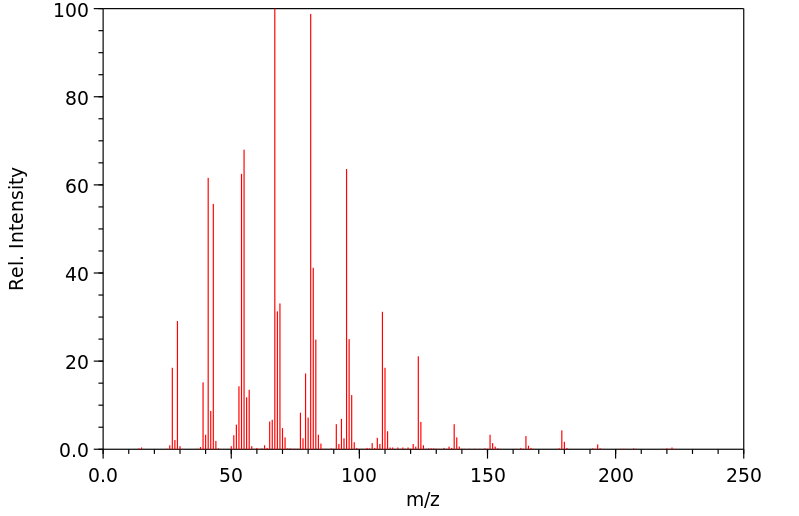
质谱MS
-
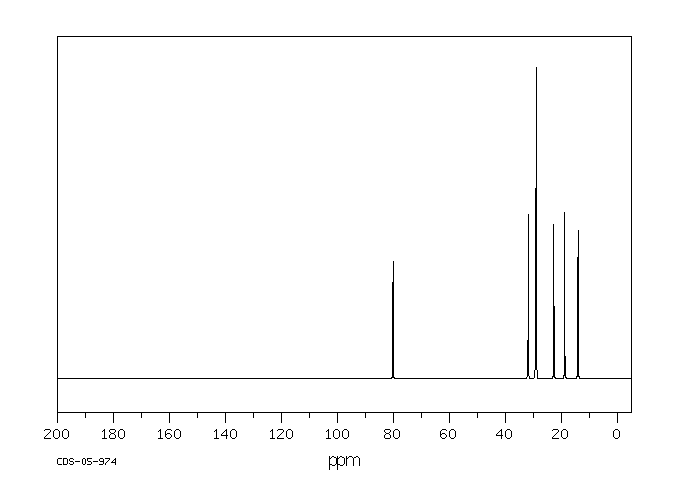
碳谱13CNMR
-
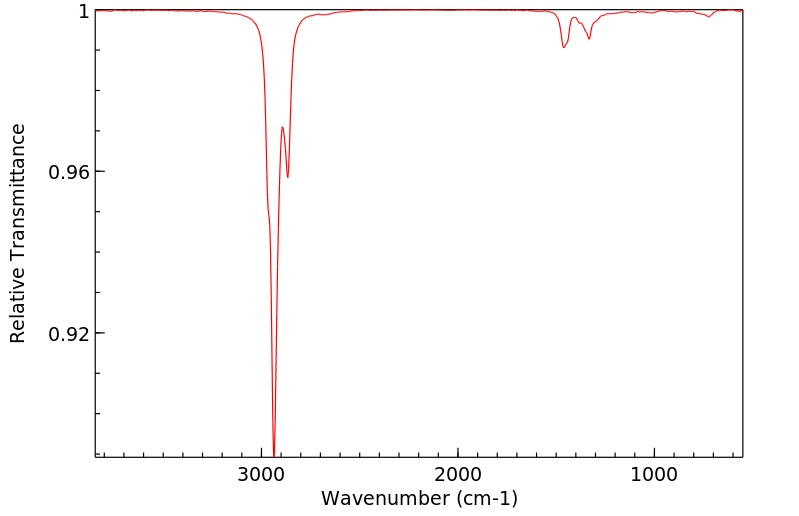
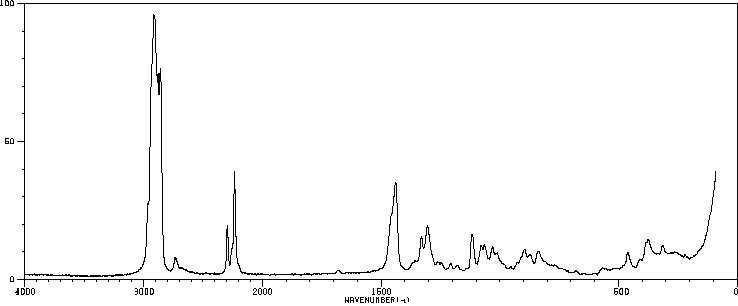
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-