3-乙酰基-6-溴香豆素 | 2199-93-1
中文名称
3-乙酰基-6-溴香豆素
中文别名
——
英文名称
3-Acetyl-6-bromo-chromen-2-one
英文别名
3-acetyl-6-bromo-2H-chromen-2-one;3-acetyl-6-bromocoumarin;6-bromo-3-acetylcoumarin;3-acetyl-6-bromochromen-2-one
CAS
2199-93-1
化学式
C11H7BrO3
mdl
MFCD00024075
分子量
267.079
InChiKey
XFQYOFLFNKCHLG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:227 °C
-
沸点:441.3±45.0 °C(Predicted)
-
密度:1.70 g/cm3
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn,Xi
-
安全说明:S26
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:2932209090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:请将药品存放在密闭、阴凉干燥的地方保存。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 3-Acetyl-6-bromocoumarin
CAS-No. : 2199-93-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Oral (Category 4)
Eye irritation (Category 2)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful if swallowed. Irritating to eyes.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H302 Harmful if swallowed.
H319 Causes serious eye irritation.
Precautionary statement(s)
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R22 Harmful if swallowed.
R36 Irritating to eyes.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C11H7BrO3
Molecular Weight : 267,1 g/mol
Component Concentration
3-Acetyl-6-bromocoumarin
CAS-No. 2199-93-1 -
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Hydrogen bromide gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 2,061
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes Causes serious eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6-bromo-3-(2-bromoacetyl)-2H-chromen-2-one 106578-01-2 C11H6Br2O3 345.975 —— 6-bromo-3-cinnamoyl-2H-chromen-2-one 80467-51-2 C18H11BrO3 355.188 —— (Z)-6-bromo-3-(3-phenylacryloyl)-2H-chromen-2-one 1354375-54-4 C18H11BrO3 355.188 —— 6-bromo-3-[(2E)-3-phenylprop-2-enoyl]-2H-chromen-2-one —— C18H11BrO3 355.188 —— 6-bromo-3-(3-p-tolylacryloyl)-2H-chromen-2-one 225367-38-4 C19H13BrO3 369.214 —— 6-bromo-3-[(2E)-3-(4-methylphenyl)prop-2-enoyl]-2H-chromen-2-one —— C19H13BrO3 369.214 —— (Z)-6-bromo-3-[3-(4-methylphenyl)acryloyl]-2H-chromen-2-one 1354375-65-7 C19H13BrO3 369.214 —— 6-bromo-3-((E)-3-(4-bromophenyl)-acryloyl)-2H-chromen-2-one 1309189-39-6 C18H10Br2O3 434.084 —— (Z)-6-bromo-3-[3-(4-bromophenyl)acryloyl]-2H-chromen-2-one 1354375-55-5 C18H10Br2O3 434.084 —— chloroacetamidomethyl 3-(6-bromocoumarinyl)ketone 117319-45-6 C13H9BrClNO4 358.576 —— 6-bromo-3-((E)-3-(4-chlorophenyl)-acryloyl)-2H-chromen-2-one 916828-87-0 C18H10BrClO3 389.633 —— dichloroacetamidomethyl 3-(6-bromocoumarinyl)ketone 117290-86-5 C13H8BrCl2NO4 393.021 —— (Z)-6-bromo-3-[3-(4-methoxyphenyl)acryloyl]-2H-chromen-2-one 916829-09-9 C19H13BrO4 385.214 —— 6-bromo-3-((E)-3-(3-chlorophenyl)-acryloyl)-2H-chromen-2-one 1309189-32-9 C18H10BrClO3 389.633 —— 6-bromo-3-[3-(4-methylthiophenyl)acryloyl]-2H-chromen-2-one 690214-42-7 C19H13BrO3S 401.28 —— 6-bromo-3-[3-(4-dimethylaminophenyl)propenoyl]chromen-2-one 690214-36-9 C20H16BrNO3 398.256 —— 3-acetyl-6-phenyl-2H-chromen-2-one 1208985-24-3 C17H12O3 264.28 6-溴香豆素-3-甲酸 6-bromocoumarin-3-carboxylic acid 2199-87-3 C10H5BrO4 269.051 —— 3-acetyl-6-(4-methylphenyl)-2H-chromenone 1208985-25-4 C18H14O3 278.307 —— 6-bromo-3-((E)-3-(2,4-dichlorophenyl)-acryloyl)-2H-chromen-2-one 916828-89-2 C18H9BrCl2O3 424.078 —— 6-bromo-3-((E)-3-(2-methoxyphenyl)-acryloyl)-2H-chromen-2-one 1309189-41-0 C19H13BrO4 385.214 —— (Z)-6-bromo-3-[3-(2-methoxyphenyl)acryloyl]-2H-chromen-2-one 1354375-57-7 C19H13BrO4 385.214 —— 3-acetyl-6-(4-chlorophenyl)-2H-chromen-2-one 1208985-27-6 C17H11ClO3 298.726 —— 3-acetyl-6-(4-fluorophenyl)-2H-chomen-2-one 1208985-28-7 C17H11FO3 282.271 —— 6-bromo-3-(3-(3-nitrophenyl)acryloyl)-2H-chromen-2-one 202828-03-3 C18H10BrNO5 400.185 —— 3-acetyl-6-(4-methoxyphenyl)-2H-chromen-2-one 1259294-11-5 C18H14O4 294.307 —— 6-bromo-3-(2-(phenylsulfonyl)acetyl)-2H-chromen-2-one 933020-73-6 C17H11BrO5S 407.241 —— 6-bromo-3-(2-tosylacetyl)-2H-chromen-2-one 932990-36-8 C18H13BrO5S 421.268 —— 2-(1-(6-bromocoumarin-3-yl)ethylidene)hydrazinecarbothioamide 187404-96-2 C12H10BrN3O2S 340.2 —— (1E)-1-(1-(6-bromo-2- oxo-2H-chromen-3-yl)ethylidene)thiosemicarbazide 1144102-26-0 C12H10BrN3O2S 340.2 —— 2-dichloroacetamido-3-hydroxy-1-<3-(6-bromocoumarinyl)>-1-propanone 117290-87-6 C14H10BrCl2NO5 423.047 —— 6-bromo-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one 80467-61-4 C17H13BrN2O2 357.206 —— (E)-6-bromo-3-(1-(2-phenylhydrazono)ethyl)-2H-chromen-2-one —— C17H13BrN2O2 357.206 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:3-乙酰基-6-溴香豆素 在 盐酸 、 溴 作用下, 以 乙醇 、 氯仿 为溶剂, 反应 9.0h, 生成 aminomethyl 3-(6-bromocoumarinyl)ketone hydrochloride参考文献:名称:氯霉素的6-溴香豆素类似物†摘要:根据Sorm法合成了dl-苏--2--2-二氯乙酰胺-1- [3-(6-溴香豆基)]丙烷-1,3-二醇(7)。报道了在电子冲击下二氯乙酰氨基甲基3-(6-溴香豆基)酮(4)的断裂。已经提出了对新合成的化合物进行体外抗菌筛选的结果。DOI:10.1002/jhet.5570250107
-
作为产物:描述:3-Acetyl-6-bromo-4-chlorochromen-2-one 在 copper(l) iodide 、 potassium carbonate 、 异丙醇 作用下, 反应 12.0h, 以84%的产率得到3-乙酰基-6-溴香豆素参考文献:名称:异丙醇中CuI对芳香族α-和β-卤代羰基化合物的区域选择性加氢脱卤摘要:在碱性条件下,使用CuI在异丙醇中开发了一种有效的且对区域选择性的芳香族α-和β-卤代羰基化合物进行加氢脱卤的方法。该反应体系可有效还原氯,溴和碘基团,并以羰基化合物形式提供高收率(最高97%)。该方法是环境友好的,并且显示出对多种电子富和取代基的优异耐受性。DOI:10.1002/ejoc.201801385
文献信息
-
One-pot multicomponent synthesis of indole incorporated thiazolylcoumarins and their antibacterial, anticancer and DNA cleavage studies作者:Rajitha Gali、Janardhan Banothu、Ramesh Gondru、Rajitha Bavantula、Yashodhara Velivela、Peter A. CrooksDOI:10.1016/j.bmcl.2014.10.100日期:2015.1A series of indole incorporated thiazolylcoumarins (7a–q) have been synthesized and evaluated for their antibacterial, anticancer and DNA cleavage studies. Analysis of antibacterial studies indicated that all the synthesized compounds possess promising activity towards the screened bacterial strains. In vitro anticancerous action was studied for compound 7a (NSC: 768621/1) against the full panel of已合成了一系列吲哚并入的噻唑基香豆素(7a - q),并对其抗菌,抗癌和DNA裂解研究进行了评估。抗菌研究的分析表明,所有合成的化合物均对筛选出的细菌菌株具有有希望的活性。研究了化合物7a(NSC:768621/1)对60种人类肿瘤细胞系的体外抗癌作用。五种剂量水平的活性结果表明,化合物7a对其中的所有细胞系均具有活性,并且已显示出对白血病的有效活性:CCRF-CEM(GI 50:0.33μM),非小细胞肺癌:NCI-H522 (GI 50:1.03μM),结肠癌:HCT-116(GI 50:1.60μM),CNS癌症:SF-539(GI 50:1.58μM),黑色素瘤MALME-3M(GI 50:1.59μM ),卵巢癌:OVCAR- 3(GI 50:1.16μM),肾癌:UO-31(GI 50:0.76μM),前列腺癌:PC-3(GI 50:0.82μM)和乳腺癌:BT-549(GI 50:1
-
Green and Rapid Access to Benzocoumarins<i>via</i>Direct Benzene Construction through Base-Mediated Formal [4+2] Reaction and Air Oxidation作者:Chengli Mou、Tingshun Zhu、Pengcheng Zheng、Song Yang、Bao-An Song、Yonggui Robin ChiDOI:10.1002/adsc.201500771日期:2016.3.3typically with an intramolecular ester forming reaction to make the lactone ring as the last step. Another major method involves transition metal‐catalyzed coupling or carbon‐hydrogen bond activation reactions starting with pre‐existing aryl frameworks in the substrates. Here we report a new strategy for the green and rapid access to benzocoumarins and their derivatives. Our method uses readily available苯并香豆素是在天然产物和合成分子中广泛发现的重要结构基序。合成苯并香豆素及其衍生物的传统方法需要多个步骤,通常需要进行分子内酯形成反应以使内酯环成为最后一步。另一种主要方法涉及过渡金属催化的偶联或碳氢键活化反应,该反应从底物中预先存在的芳基骨架开始。在这里,我们报告了绿色和快速获得苯并香豆素及其衍生物的新策略。我们的方法使用容易获得的不饱和醛和香豆素作为底物,使用空气作为绿色氧化剂。整个反应通过正式的[4 + 2]过程进行,以构建新的苯环,从而基本上在单个步骤中得到苯并香豆素。没有使用金属催化剂。不涉及有毒或昂贵的试剂。我们的新方法的强大功能在大麻素(一种具有生物活性的天然产物)的简明正式全合成中得到了进一步证明。
-
Design and synthesis of novel carbazolo–thiazoles as potential anti-mycobacterial agents using a molecular hybridization approach作者:Mahamadhanif S. Shaikh、Mahesh B. Palkar、Harun M. Patel、Rajesh A. Rane、Wesam S. Alwan、Mahidansha M. Shaikh、Iqbal M. Shaikh、Girish A. Hampannavar、Rajshekhar KarpoormathDOI:10.1039/c4ra11752b日期:——
A series of novel carbazolo–thiazoles was synthesized and evaluated for
in vitro anti-mycobacterial activity. -
Proton transfer process in synthesis of 3-acetyl-4-(substituted ethylenyl)coumarins and chromeno[3,4-c]pyridines作者:Abdolali Alizadeh、Reza Mohammadi、Fahimeh Bayat、Long-Guan ZhuDOI:10.1016/j.tet.2018.03.015日期:2018.43-acetylcoumarins in dichloromethane afford functionalized 3-acetyl-4-(substituted ethylenyl)coumarins. Also, triphenylphosphine-catalyzed three-component cascade annulation reactions of 3-acetylcoumarins, activated acetylenic compounds, and hydrazines or amines provide a straightforward access to 3,5-dihydro-2H-chromeno[3,4-c]pyridine-1,2-dicarboxylates. In these strategies, the main step to the target products
-
Discovery of New Coumarin-Based Lead with Potential Anticancer, CDK4 Inhibition and Selective Radiotheranostic Effect: Synthesis, 2D & 3D QSAR, Molecular Dynamics, In Vitro Cytotoxicity, Radioiodination, and Biodistribution Studies作者:Mona O. Sarhan、Somaia S. Abd El-Karim、Manal M. Anwar、Raghda H. Gouda、Wafaa A. Zaghary、Mohammed A. KhedrDOI:10.3390/molecules26082273日期:——MCF-7, A-549, and CHO-K1 cell lines, respectively. The CDK4 enzyme assay revealed the significant CDK4 inhibitory activity of compound 2b with IC50 of 0.036 µM. The selectivity of the newly discovered lead compound 2b toward localization in tumor cells was confirmed by a radioiodination biological assay that was done via electrophilic substitution reaction utilizing the oxidative effect of chloramine-t合成了基于6-溴香豆素-亚乙基-肼基-噻唑基和6-溴香豆素-噻唑基的衍生物。具有高预测能力r 2 = 0.92和RMSE = 0.44的定量结构活性关系(QSAR)模型预测了5种化合物;图2b,3b,5a,9a和9i具有潜在的抗癌活性。化合物2b的最佳ΔG为–15.34 kcal / mol,亲和力为40.05 pki。在分子动力学研究中,2b显示在3.5 ns后达到0.8Å的平衡,而黄酮哌啶醇在同一时间(3.5 ns)后达到0.5Å的平衡。图2b显示了IC 50分别针对MCF-7,A-549和CHO-K1细胞系的0.0136 µM,0.015 µM和0.054 µM。CDK4酶分析显示化合物2b具有显着的CDK4抑制活性,IC 50为0.036 µM。通过放射性碘化生物测定法证实了新发现的先导化合物2b对肿瘤细胞定位的选择性,所述放射性碘化生物测定法通过利用氯胺-t的氧化作用的亲电取代反应进行。131
表征谱图
-
氢谱1HNMR
-
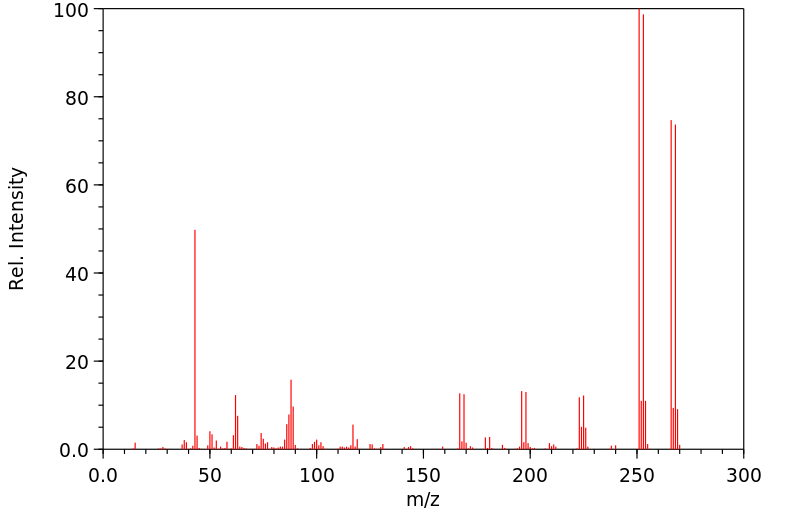
质谱MS
-
碳谱13CNMR
-
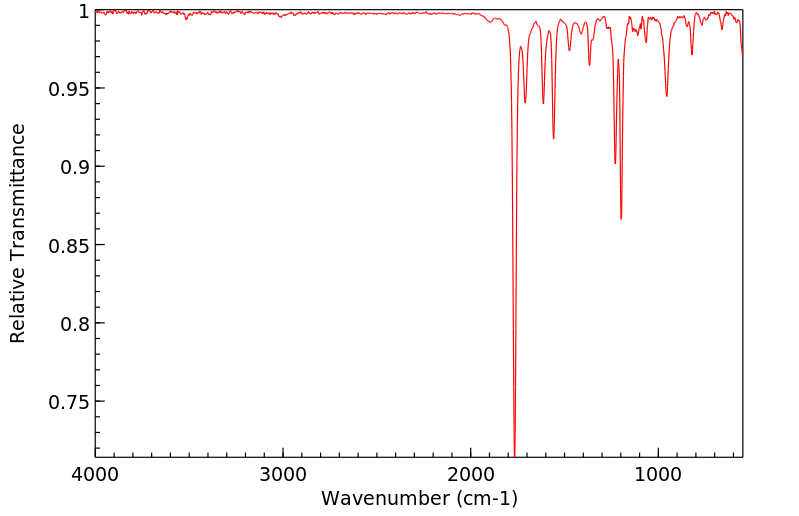
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯