2,4',5-三氯联苯醚 | 16606-02-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:67℃
-
沸点:334.36°C (rough estimate)
-
密度:1.3510 (rough estimate)
-
溶解度:5.55e-07 M
-
蒸汽压力:4.00e-04 mmHg
-
亨利常数:1.90e-04 atm-m3/mole
-
大气OH速率常数:1.20e-12 cm3/molecule*sec
-
保留指数:1829;1818;1846;1819;1820;1839.2;1848.8;1858;1858.1;1819
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
危险品标志:N
-
安全说明:S35
-
危险类别码:R33
-
海关编码:2903999090
-
危险品运输编号:UN 3432 9/PG 2
-
储存条件:| 室温 |
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:环氧改性钴羰基催化剂催化芳基卤化物羰基化合成芳族羧酸摘要:开发了一种新方法,用于通过相应的芳基卤化物的羰基化合成芳族和杂芳族酸及其衍生物(酯,盐)。在非常温和的条件下可以高产率选择性地形成酸。高活性的催化体系(用环氧化物改性的含羰基钴钴的含碱醇溶液)用于活化芳基卤化物。DOI:10.1007/s11167-005-0619-y
-
作为产物:描述:参考文献:名称:Bellavita, Gazzetta Chimica Italiana, 1935, vol. 65, p. 632,643摘要:DOI:
文献信息
-
Experimental and theoretical studies on synthesis and structure elucidation of some polychlorinated biphenyl derivatives作者:Vadim P. Boyarskiy、Irina А. Boyarskaya、Elisaveta A. Savicheva、Maria Gdaniec、Marina S. Fonari、Yurii A. SimonovDOI:10.1016/j.molstruc.2010.04.019日期:2010.64-chloro-2-(4-chlorophenyl)benzoate 2 . The substitution of methoxycarbonyl group for chlorine in ortho -position of trichlorobiphenyls seems not to affect the twist angle of the biphenyl unit. However, the twist angle of the methoxycarbonyl group relative to the phenyl ring was found to be significantly larger in the meta -derivative 1 than the ortho -derivative 2 . To rationalize conformational differences
-
Synthesis of polychlorinated biphenyls and their metabolites with a modified Suzuki-coupling作者:Izabela Kania-Korwel、Sean Parkin、Larry W Robertson、Hans-Joachim LehmlerDOI:10.1016/j.chemosphere.2004.04.035日期:2004.8A modified procedure for the synthesis of polychlorinated biphenyls (PCBs) utilizing the Suzuki-coupling, a palladium-catalyzed cross-coupling reaction, is described. The coupling of (chlorinated) benzene boronic acids with bromochlorobenzenes, using Pd(dppf)(2)Cl(2) (dppf = 1,1'-bis(diphenylphosphino)ferrocene) as the catalyst and aqueous sodium carbonate as the base, gave the desired PCB congeners描述了使用铃木偶联(钯催化的交叉偶联反应)合成多氯联苯(PCB)的改进方法。以Pd(dppf)(2)Cl(2)(dppf = 1,1'-双(二苯基膦基)二茂铁)为催化剂,碳酸钠水溶液为碱,将(氯化)苯硼酸与溴氯苯偶合所需的PCB同类产品,产量适中至良好。使用此修改程序合成了11种PCB同类物,包括对环境重要的PCB同类物和代谢物。与Pd(PPh(4))(4)相比,这种新的催化剂Pd(dppf)(2)Cl(2)具有空气敏感性较低的优点,并且具有更长的保存期限。通过将氯化苯硼酸与溴(二)甲氧基苯偶联或通过将(二)甲氧基苯硼酸与氯化溴苯偶联,使用相同的步骤合成了三个新的(二)甲氧基化PCB同类物。使用三溴化硼的二氯甲烷溶液可以轻松地将二甲氧基化的PCB同类物转化为相应的二羟基化的PCB衍生物。与常规方法(例如Cadogan反应)相比,该方法具有高选择性和中等至良好收率的优势,并允许使用毒性较小的原料。
-
Photochemical behaviour of 1,4-dichlorobenzene in aqueous solution作者:Laurence Meunier、Jean-François Pilichowski、Pierre BouleDOI:10.1139/v01-091日期:2001.7.1
Several photoproducts were identified in the direct photolysis of 1,4-dichlorobenzene (1,4-DCB) in air-saturated aqueous solution, namely 4-chlorophenol, hydroquinone, hydroxybenzoquinone, and 2,5-dichlorophenol. In the absence of oxygen the latter is not formed and phenol was detected, but the unexpected formations of 4,4'-dichlorobiphenyl, 2,4',5-trichlorobiphenyl, and a terphenyl derivative are observed. Mechanisms are proposed to explain the formations of identified photoproducts. The phototransformation of 1,4-DCB may be photoinduced by NO3 or FeIII salts. The main primary product is 2,5-dichlorophenol, which results from a hydroxylation without dechlorination. Some other products have been identified in particular 4-chlorophenol and 2,5-dichlorobenzoquinone in the case of FeIII salts.Key words: 1,4-dichlorobenzene, photolysis, aqueous solution, induced phototransformation.
-
Instruments for detecting low-molecular weight substance申请人:Mizukami Haruki公开号:US20050148097A1公开(公告)日:2005-07-07To provide the following instruments 1 and 2 as a low-molecular-weight substance detection instrument employing immunochromatography capable of detecting conveniently and sensitively detecting a low-molecular weight substance such as an environmental pollutant (e.g., a dioxin), as a target substance contained in a test sample: 1. an instrument, which comprises 1) a test sample application section with which a test sample is brought into contact; 2) a label product reaction section containing a label product containing, as a portion thereof, an antibody capable of binding to a target substance contained in the test sample, the label product being not bound to the reaction section; 3) an unbound label product capturing section containing an element capable of capturing the label product which is not bound to the target substance, the element being bound to the capturing section; and 4) a detection section containing a detection element which, when coming into contact with the target substance bound to the label product, causes a visually observable change, and 2. an instrument wherein a test sample is reacted with a labeled antibody containing, as a portion thereof, an antibody capable of binding to a target substance contained in the test sample, and the resultant reaction product is employed for detecting the target substance contained in the test sample.提供以下仪器1和2作为低分子量物质检测仪器,采用免疫层析技术,能够方便敏感地检测低分子量物质,例如环境污染物(例如二恶英),作为测试样品中包含的目标物质:1. 仪器,包括1)测试样品应用部分,用于将测试样品接触;2)标签产物反应部分,包含一种标签产物,其中包含一种能够结合到测试样品中的目标物质的抗体,标签产物未结合到反应部分;3)未结合标签产物捕获部分,包含一种能够捕获未结合到目标物质的标签产物的元素,该元素与捕获部分结合;以及4)检测部分,包含一种检测元素,当与结合到标签产物的目标物质接触时,会引起可视的变化;2. 仪器,其中测试样品与标记抗体反应,其中一部分包含能够结合到测试样品中的目标物质的抗体,产生的反应产物用于检测测试样品中包含的目标物质。
-
Generation of aryl radicals from <i>in situ</i> activated homolytic scission: driving radical reactions by ball milling作者:Xinjie Yang、Hao Wang、Yanhua Zhang、Weike Su、Jingbo YuDOI:10.1039/d2gc00910b日期:——The need for an operationally straightforward application of radical chemistry has led researchers to explore practical strategies to obtain and trap radicals. Herein, we report a mechanoradical generation method by direct C–N bond breaking of aryldiazonium salts using NaCl as an activator. This method opens a new pathway to radical reactions ranging from (hetero)arylation, cascade addition, and hydrogen
表征谱图
-
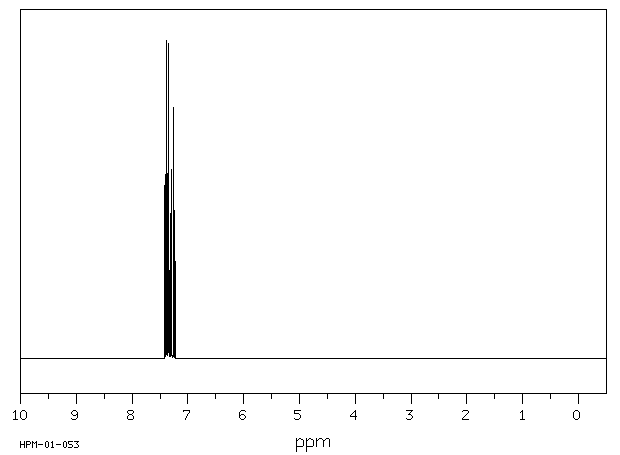
氢谱1HNMR
-
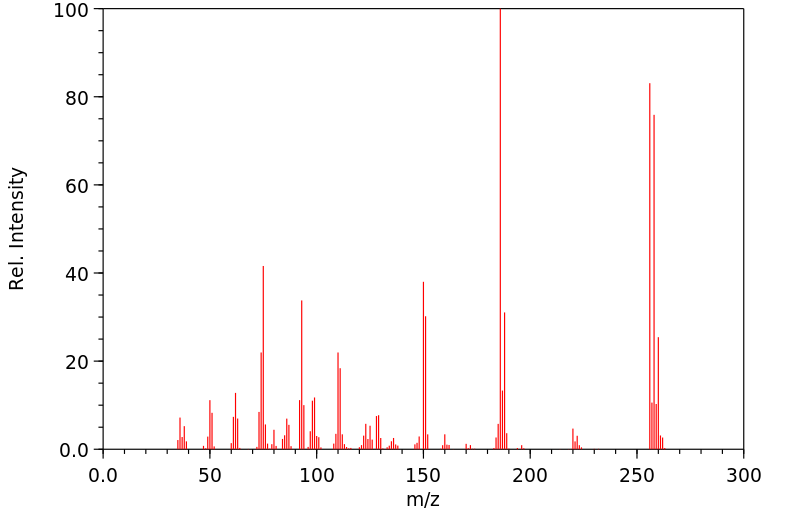
质谱MS
-
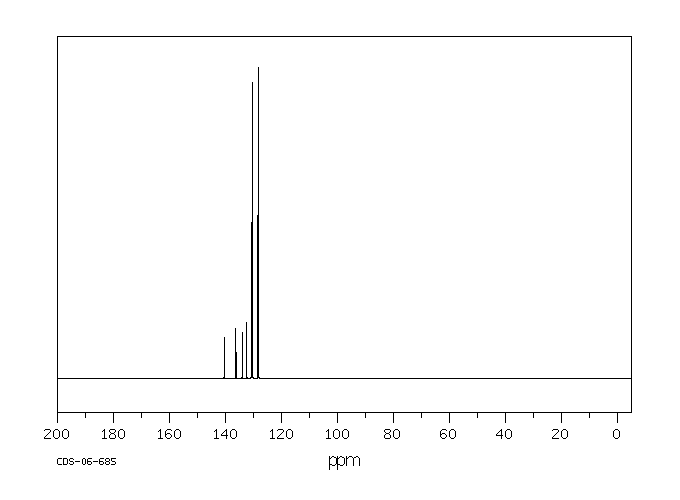
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息