3,4'-二氯联苯 | 2974-90-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:125 °C(Press: 1 Torr)
-
密度:1.249±0.06 g/cm3(Predicted)
-
保留指数:1737;1764
计算性质
-
辛醇/水分配系数(LogP):5.2
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4',5-三氯联苯醚 2,4',5-Trichlorobiphenyl 16606-02-3 C12H7Cl3 257.547
反应信息
-
作为反应物:描述:参考文献:名称:Nickel-on-Charcoal-Catalyzed Reductions of Aryl Chlorides摘要:将(功能化)芳基氯化物暴露于催化量的碳灰镍,并在恒温回流的乙腈中加入化学计量的 Me2NH · BH3/K2CO3,可以得到高产率的还原芳烃。在这些条件下,PCB 也能被还原。该方法对水分的耐受性非常高。DOI:10.1055/s-2001-14636
-
作为产物:描述:参考文献:名称:BEADLE, J. R.;KORZENIOWSKI, S. H.;ROSENBERG, D. E.;GARCIA-SLANGA, B. J.;G+, J. ORG. CHEM., 1984, 49, N 9, 1594-1603摘要:DOI:
文献信息
-
Visible-Light-Promoted, Catalyst-Free Gomberg–Bachmann Reaction: Synthesis of Biaryls作者:Juyoung Lee、Boseok Hong、Anna LeeDOI:10.1021/acs.joc.9b00557日期:2019.7.19Biaryls were synthesized via a novel visible-light-promoted Gomberg–Bachmann reaction that does not require a photosensitizer or any metal reagents. The formation of an electron donor–acceptor complex between aryl diazonium salts and pyridine allows, under visible-light irradiation, the synthesis of biaryls in moderate-to-high yields.
-
Aryl radicals from hexazadienes and tetrazenes作者:Donald Mackay、Deane Douglas McIntyreDOI:10.1139/v82-148日期:1982.4.15
Aryl radicals are produced from both ends of the hexazadienes 1 and 2 and from the tetrazene 3, either thermally or photolytically. They attack aromatic compounds in the nucleus, the yield of biaryl being in the range 40–70%, though it can be made nearly quantitative by using m-dinitrobenzene as an additive. The aryl radicals also oxidize 2-propanol to acetone, the reduction products being the halogenobenzene and 1,2-diacetylhydrazine.Photolysis of 1 goes mainly by way of the tetrazene 3, and this may also be a significant pathway in the thermal reaction. Azodiacetyl is an intermediate in the thermal reaction of 1 with 2-propanol and may be generally so in all its reactions.Radical induced decomposition is believed to be important in the reactions of 1, 2, and 3, and it is probably responsible for the formation of 1-acetyl-1-arylhydrazines, routinely produced in yields of up to 25%.
-
Hydrocarbons and chloroaromatics from anilines and n-butyl nitrite作者:Angelo G. Giumanini、Giancarlo Verardo、Fausto Gorassini、Paolo StrazzoliniDOI:10.1002/recl.19951140703日期:——A single reagent, i.e. n-butyl nitrite, can be used to oxidize an aromatic amine, or the corresponding N-methylene derivative, to a diazo compound followed by its subsequent reduction to hydrocarbon in a single batch. Alternatively, a chloro derivative can be obtained if carbon tetrachloride is used as the solvent. The reactions appear to be general and complete product identification was accomplished
-
Palladium(II)-catalyzed oxidative coupling of arenes by thallium(III)作者:Anatoly K. Yatsimirsky、Sergei A. Deiko、Alexander D. RyabovDOI:10.1016/s0040-4020(01)91964-7日期:1983.1thallium(III) trifluoroacetate in the presence of catalytic amounts of palladium(II) acetate affords biaryls in good yields. The GLC study of the isomer distribution has shown that 4,4 '-biaryls are the major products. Thus, the pure 4,4 '-biaryls can be easily isolated either by recrystallization or column chromatography. The competitive experiments and kinetic study using arenes and arylthallium derivatives在催化量的乙酸钯(II)存在下,通过三氟乙酸fluoro(III)用供电子键和中度吸电子取代基氧化苯,可得到高产率的联芳基。对异构体分布的GLC研究表明,4,4'-联芳基是主要产物。因此,可以通过重结晶或柱色谱法容易地分离出纯的4,4'-联芳基。使用芳烃和芳基al衍生物作为起始原料的竞争性实验和动力学研究以及淬灭实验表明,反应的第一步是芳烃的快速th化以形成芳基intermediate中间体ArTl(OOCCF 3)2。后者经历与三聚体Pd 3(OAc)6解聚形成的单体络合物Pd(OAc)2反应的速率确定性金属转移步骤。随后芳基钯物质的快速分解得到最终的反应产物。芳烃的盐酸盐化和用ArTl(OOCCF 3)2中的Pd II取代Tl III的特征分别是Hammett图的斜率分别为-5.6(XXX +)和-3.0(XXX)。
-
CARBAZOLE COMPOUND AND USE THEREOF申请人:Matsumoto Naoki公开号:US20120203010A1公开(公告)日:2012-08-09A carbazole compound represented by the following formula: wherein, when m=1, n=0, Ar 1 , Ar 2 , Ar 3 and X 2 are C 6-50 aryl or C 4-50 heteroaryl, provided that Ar 1 and Ar 2 , or Ar 3 and X 2 may form together a ring; X 1 ═C 6-50 arylene; R 1 , R 2 , R 4 , R 5 and R 7 are H, halogen, amino, C 1-18 alkyl, C 1-18 alkoxy, C 6-50 aryl or C 4-50 heteroaryl, R 3 and R 6 are H, halogen, C 1-18 alkyl, C 1-18 alkoxy, C 6-50 aryl or C 4-50 heteroaryl; when m=0, n=1-3, Ar 3 , Ar 4 and Ar 5 are C 6-50 aryl or C 4-50 heteroaryl, Ar 4 and Ar 5 may form together a ring; X 1 ═C 1-18 alkyl, C 6-50 aryl or C 4-50 heteroaryl; X 2 ═C 6-50 arylene; R 1 -R 7 are H, halogen, C 1-18 alkyl, C 1-18 alkoxy, C 6-50 aryl or C 4-50 heteroaryl; when m=0, n=0, X 1 ═C 1-18 alkyl, C 6-50 aryl or C 4-50 heteroaryl; Ar 3 and X 2 are C 6-50 aryl or C 4-50 heteroaryl; R 2 ═H, halogen, C 1-18 alkyl, C 1-18 alkoxy; R 1 and R 3 -R 7 are H, halogen, C 1-18 alkyl, C 1-18 alkoxy, C 6-50 aryl or C 4-50 heteroaryl. The carbazole compound is suitable for an organic EL device.以下是该化合物的中文翻译:其中,当m=1,n=0时,Ar1、Ar2、Ar3和X2为C6-50芳基或C4-50杂环芳基,前提是Ar1和Ar2,或Ar3和X2可能共同形成一个环;X1=C6-50芳基;R1、R2、R4、R5和R7为H、卤素、氨基、C1-18烷基、C1-18烷氧基、C6-50芳基或C4-50杂环芳基,R3和R6为H、卤素、C1-18烷基、C1-18烷氧基、C6-50芳基或C4-50杂环芳基;当m=0,n=1-3时,Ar3、Ar4和Ar5为C6-50芳基或C4-50杂环芳基,Ar4和Ar5可能共同形成一个环;X1=C1-18烷基、C6-50芳基或C4-50杂环芳基;X2=C6-50芳基;R1-R7为H、卤素、C1-18烷基、C1-18烷氧基、C6-50芳基或C4-50杂环芳基;当m=0,n=0时,X1=C1-18烷基、C6-50芳基或C4-50杂环芳基;Ar3和X2为C6-50芳基或C4-50杂环芳基;R2=H、卤素、C1-18烷基、C1-18烷氧基;R1和R3-R7为H、卤素、C1-18烷基、C1-18烷氧基、C6-50芳基或C4-50杂环芳基。这种咔唑化合物适用于有机EL器件。
表征谱图
-
氢谱1HNMR
-
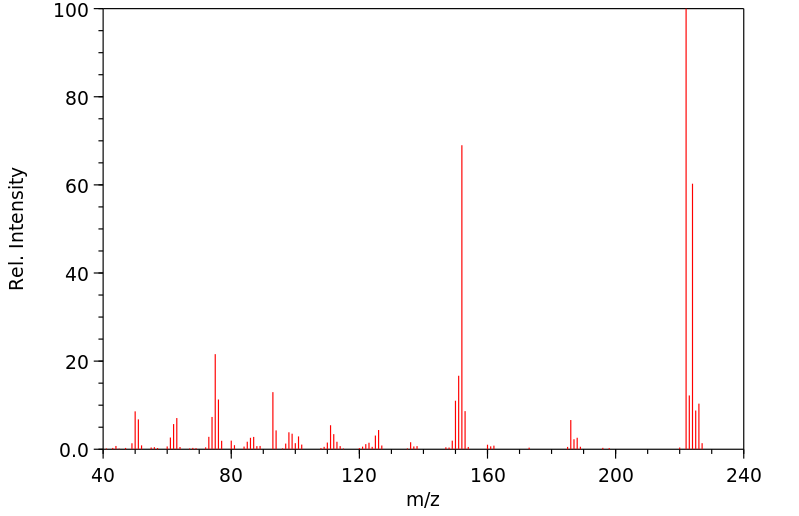
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息